This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
Weiler Labeling Systems (WLS), a ProMach product brand, introduces their AutonomyTM IV on-demand digital label printer at Pack Expo Las Vegas 2019 in both the Healthcare Packaging Expo booth #N-307 and in the Main Hall booth #C-3225. Responding to increased customer interest as well as a variety of newly identified applications, WLS has refined the Autonomy IV printer to meet these new needs in pharmaceutical and non-pharmaceutical markets.
WLS’s Autonomy IV is ideal for customers looking for quick turnaround, production planning flexibility, real-time variable data application, inventory reduction and/or multi-lingual label printing. When combined with one of the various vision inspection options, such as the Total Layout Control (TLC) system from Antares Vision, the customer achieves quality control of their label printing operation.
To further introduce Autonomy IV to the marketplace, WLS and Antares Vision will present the features and capabilities of this advanced printer on the Innovation Stage at Pack Expo Las Vegas in the Main Hall, Innovation Stage 3 (C-1041) on Tuesday, September 24th at 1:00 PM. In this setting, attendees have an opportunity to see an informative presentation on how print-on-demand labeling and whole-label inspections are transforming the packaging market and then engage in a Q&A session.
With all of its sophisticated capabilities, Autonomy IV can still be operated with minimal training. Printing can be initiated locally on the printer with artwork stored on its hard drive, or it can be connected to a network for greater flexibility. In addition, it can be connected directly to a line data management system for even greater capability and/or control.
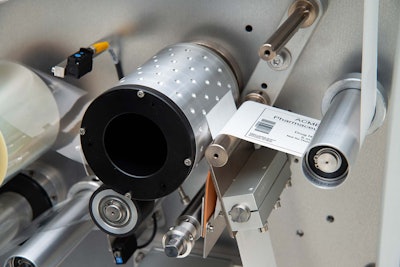
Featuring a UV-curing, drop-on-demand (DOD) printer, Autonomy IV can print most barcode formats and human readable codes whether from static or dynamic data, including serialized data. In addition, it can print high-impact visual graphics in full color or black-and-white at speeds up to 3,050 inches (77 m) per minute. Applications include printing variable data on pre-printed labels or on-demand printing the entire label from ‘bright stock,’ as needed. Achieving superior print quality and print wear resistance on many substrates without the need for a protective top coat, Autonomy IV is capable of producing the highest graded codes. It can handle a label roll size up to 18 inches (457 mm) in diameter with a 3-inch (76-mm) diameter core.
WLS supports their Autonomy series of label printers with an industry-leading warranty and world-class technical service. WLS provides inks for Autonomy printers and supports customers in identifying the appropriate substrate for their needs.