In a consumer-driven world where recalls for food and cars are immediately blared in emails, letters, TV commercials, and other sources, it is surprising (and yet no secret) that a similarly effective system is not put in place for medical device recalls, despite that these recalls affect the lives of many U.S. citizens.
As of July 23 of this year, there have been 30 recalled devices, all identified as Class I recalls—the most critical kind as the use of these devices can result in serious injuries or death—by the U.S. Food and Drug Administration (FDA). To put things in perspective, 32 Class I recalls total were sent out in 2018. The FDA reports that recalls affect more than 750 devices, which is nearly 50 million individual devices in the U.S.
However, the number of recalls featured in these kinds of lists often differs due to:
- Some Class I recalls are not being included in the FDA’s annual lists.
- Some of the entries on the list account for more than one database entry when the recall affects multiple models made by a company.
These discrepancies concerning information in the medical device supply chain add to the difficulties patients already have in learning about these recalls and result in many patients never being informed.
A recent blog post by Stericycle Services’ ExpertSolutions titled “Medical Device Trends and Why They Make for Tough Tracking” explains that one of the biggest challenges of industry is being able to quickly and effectively track recalled product. “It lags behind in its capacity to alert patients about safety issues,” says ExpertSolutions. “For the tens of millions of patients with implantable devices, they must keep their own records and then check manufacturing codes and dates of recalled products against theirs.”
Attempts have been made to fix this issue. The FDA started digitizing its records a decade ago and released its openFDA, a collection of databases that house information. However, only some of the databases cross-reference. Final Rule was also issued by the FDA requiring medical devices to have a unique device identifier (UDI) which would provide information such as the manufacturer, the specific device model, and its lot and serial numbers so that companies can more easily determine the scope of the recall.
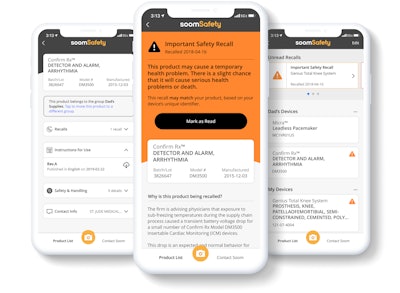
Now Soom, a mission-based organization set on connecting medical device manufacturers, healthcare providers, home care providers, and patients, has launched an iOS mobile app called SoomSafety to bridge the gaps between sources of important healthcare information.
How it works
“How recalls happen and how people find out about them is not proactive enough,” says Charlie Kim, President and Chief Executive Officer of Soom. When medical device manufacturers realize there is a problem with a device, they will give a press release which is then passed along to the FDA who puts the information on openFDA, but it is not guaranteed that individuals using those devices will be notified.
Kim experienced this firsthand when his youngest daughter, who was born with an airway disorder requiring her to rely on a tracheostomy tube to breathe, suddenly stopped breathing and had to be rushed to the hospital. Thankfully, she survived the episode, and Kim was determined to find out what the cause of the terrible incident had been. He discovered that the tracheostomy tube had been recalled and shouldn’t have been in use. This inspired a personal mission which led to the foundation of Soom and creating the SoomSafety app to ensure no one had to go through what his family had lived.
“When you are told to go home and have a mini hospital in the home to which people are mailing you medical devices and drugs on a daily basis, wouldn’t you presume everything is safe?” asks Kim. “At hospitals, you have facilities people making sure everything is safe, but when you are at home, that’s not the case. You as a parent are the center of healthcare, the coordinating specialist. The only thing you don’t have the ability to quickly figure out is whether everything in your mini hospital is safe to use on a constant basis.”
To meet the needs of these individual centers of healthcare, Soom has utilized barcode and knowledge graph technologies to create SoomSafety, which interconnects the openFDA databases as well as some private databases from the manufacturers. The app is designed to store device information and then sift and sort through all of the databases to find relevant information for the end-users, such as user instructions and current safety and recall information. It is created to push notifications if a device has been recalled and provide next steps. All consumers have to do is use the app to scan the UDI barcodes on a medical device or its identification card. Because of the UDI standard, no additional barcodes need to be added by the manufacturers and no upgrades or new regulation compliance will affect the app’s scanning abilities.
Much like a Google search, scanning the barcodes performs a ‘Soom search’ where, on the righthand side, you’ll find a summary of all the most accurate, relevant, and timely information, and lists of sources on the left, according to the company. The same process goes for both clients and manufacturers, who should be able to verify what a machine is and match it to their invoices and POs.
The App, the FDA, and the future
The company believes that access to recall and other information on medical devices—which individuals are trusting to improve their health and, in some cases, keep them alive—should be free for consumers, so SoomSafety is freely available and opensource. Soom’s revenue is generated on the other end of the supply chain.
Manufacturers pay the company to filter, sort and rank information and assure its accuracy and compliancy to regulations. Soom’s knowledge graph platform resolves data conflicts to build trust between two parties and their information, and in this case between a manufacturer’s massive enterprise resource planning (ERP) systems, databases, and software systems. The company uses technology and human interaction at a scale.
Also, SoomSafety’s future-proofing method includes the app being cloud-based, designed for any updates or maintenance to be pushed immediately to all subscribers, so no device contains partial or old information.
Kim says the FDA also indirectly gives the thumbs up to Soom, as individuals who are part of the FDA and both retired and current members of the Center of Devices and Radiological Health (CDRH) review SoomSafety and use the company as an example at conferences for what a collaboration between the FDA and private organizations can accomplish. Some retired members of the FDA and CDRH even partner with Soom on its advisory board through their own private industries.
Soom’s future plans include a push for updating pharmaceutical drugs into the app and spreading its reach to a more global population. The company has already incorporated the U.S. UDI rules, is working very closely on a five to 10-year project with the EU’s new medical device regulations known as the EUMDR, and is starting to talk about Saudi Arabia, Turkey, China, and Japan.
“We pay attention to the rules and regulations of each, and when they are close to being finalized, we will make sure that we develop them into our system and have them ready to go, then push them immediately. So, all of our subscribers will be at the cutting-edge of these