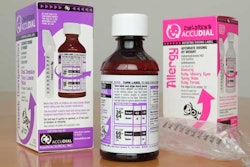
The two companies partner to provide serialization for track and trace and diversion control, offering unit-level product identification via variable data printing of 2D barcodes and human-readable codes. They also provide authentication solutions for anti-counterfeiting through development of labels and cartons marked with proprietary multi-wavelength taggants and invisible inks for authentication in the field by trading partners, consumers, and law enforcement.
COVECTRA and MSO have developed advanced integrated solutions for the pharmaceutical, tobacco and beverage industries, among others, which effectively address Europe's new Falsified Medicines Directive, and overall security issues within the supply chain. The solutions, which combine MSO's advanced packaging products with COVECTRA's technologies and services, combat counterfeiting, diversion, and return-management issues by offering manufacturers the ability to track their products from distributor to wholesaler, through to the retailer, and to the consumer.
The European directive reflects an agreement reached with the European Parliament during the Belgian presidency (headed by Acting Prime Minister, Yves Leterme). It requires authenticity features on prescription drug packaging, allowing verification of individual packs throughout the supply chain. It also requires strengthened requirements for control and inspection of manufactures of active substances, regardless of where ingredients are manufactured. Additionally, manufactures must now report any suspicion of falsified medicines; and the initiative places provisions on the sales of medicines via the Internet. Penalties will now be instituted for the manufacturing distribution, import and export of any falsified medicinal products, and member states have approximately 18 months in which to transpose the new rules into national law.
“This newly developed directive by the European government is a first step in protecting consumers and saving pharmaceutical companies millions. The initiative will certainly put pressure on the US government to continue to aggressively address what is now being referred to as an epidemic,” stated COVECTRA president, Steve Wood. “Working with a high-end packaging materials company such as MSO, we're able to provide a whole-product solution including security assessments, product and brand strategy implementation plans, security label design, as well as printing equipment and packaging material, allowing us to offer clients multiple layers of security protection, as well as extend our reach creating a two-way security solutions distribution channel,” he added.
COVECTRA's flagship product AuthentiTrack£ provides unprecedented capabilities enabling unique identification down to the unit dose that can be leveraged for complete supply chain visibility, product integrity and track and trace applications. Working together with companies like MSO, which offer extensive experience in printing and packaging line integration, high volume transaction processing and product security techniques, AuthentiTrack£ creates a platform by which applications targeting specific business problems can be rapidly deployed. The Patient Tracking, Abuse and Diversion Monitoring module is an AuthentiTrack£ native application designed to provide insight and control to pharmaceutical brand owners of abused and misused products.
“Serialization technology offered by companies like COVECTRA will speed up the securing of the opioid supply chain for a very low cost investment to brand owners,” stated MSO Chief Executive Officer, Dominic Walsh. “Serialization easily provides a way to track and trace the authenticity, safety, and verification of the drug user; working with COVECTRA we're able to provide our customers the most user friendly item code and traceability history. Together we offer a solution for product security and brand integrity, to continue the ongoing battle against counterfeiting and diversion control,” he added.
COVECTRA and MSO have developed advanced integrated solutions for the pharmaceutical, tobacco and beverage industries, among others, which effectively address Europe's new Falsified Medicines Directive, and overall security issues within the supply chain. The solutions, which combine MSO's advanced packaging products with COVECTRA's technologies and services, combat counterfeiting, diversion, and return-management issues by offering manufacturers the ability to track their products from distributor to wholesaler, through to the retailer, and to the consumer.
The European directive reflects an agreement reached with the European Parliament during the Belgian presidency (headed by Acting Prime Minister, Yves Leterme). It requires authenticity features on prescription drug packaging, allowing verification of individual packs throughout the supply chain. It also requires strengthened requirements for control and inspection of manufactures of active substances, regardless of where ingredients are manufactured. Additionally, manufactures must now report any suspicion of falsified medicines; and the initiative places provisions on the sales of medicines via the Internet. Penalties will now be instituted for the manufacturing distribution, import and export of any falsified medicinal products, and member states have approximately 18 months in which to transpose the new rules into national law.
“This newly developed directive by the European government is a first step in protecting consumers and saving pharmaceutical companies millions. The initiative will certainly put pressure on the US government to continue to aggressively address what is now being referred to as an epidemic,” stated COVECTRA president, Steve Wood. “Working with a high-end packaging materials company such as MSO, we're able to provide a whole-product solution including security assessments, product and brand strategy implementation plans, security label design, as well as printing equipment and packaging material, allowing us to offer clients multiple layers of security protection, as well as extend our reach creating a two-way security solutions distribution channel,” he added.
COVECTRA's flagship product AuthentiTrack£ provides unprecedented capabilities enabling unique identification down to the unit dose that can be leveraged for complete supply chain visibility, product integrity and track and trace applications. Working together with companies like MSO, which offer extensive experience in printing and packaging line integration, high volume transaction processing and product security techniques, AuthentiTrack£ creates a platform by which applications targeting specific business problems can be rapidly deployed. The Patient Tracking, Abuse and Diversion Monitoring module is an AuthentiTrack£ native application designed to provide insight and control to pharmaceutical brand owners of abused and misused products.
“Serialization technology offered by companies like COVECTRA will speed up the securing of the opioid supply chain for a very low cost investment to brand owners,” stated MSO Chief Executive Officer, Dominic Walsh. “Serialization easily provides a way to track and trace the authenticity, safety, and verification of the drug user; working with COVECTRA we're able to provide our customers the most user friendly item code and traceability history. Together we offer a solution for product security and brand integrity, to continue the ongoing battle against counterfeiting and diversion control,” he added.
Companies in this press-release