Thirty stockkeeping units. Global markets. Sophisticated product chemistry. Easy-to-use tester. And, especially, protection from moisture. “Moisture is the enemy,” states Ralph Hanna, director of materials, LXN Corp., San Diego, CA.
These complex, diverse requirements distill down to one primary packaging solution for LXN for the test strips that allow diabetics to self-monitor blood sugar levels: DesiCap® desiccator caps and vials from Multisorb Desiccants (Buffalo, NY).
According to Hanna, the primary packaging part of the development was straightforward. “We supply test strips for two different tests that must be kept moisture-free to get an accurate reading,” he says. “If the strips weren’t desiccated, [their accuracy] wouldn’t last for more than a week. With desiccation, the shelf life is about a year. Our packaging and production was designed around the DesiCap and vial in order to maximize shelf life. No other packaging was ever considered.”
LXN manufactures two types of test strips, a glucose test strip and a fructosamine test strip, which require similar protection from moisture. Both products use the same DesiCap and vial, but they are distinguished graphically by green and blue labels.
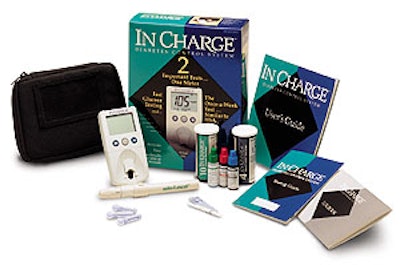
The strip-containing vials are a crucial part of LXN’s “In Charge” diabetes monitoring system, a product line anchored by a cartoned kit that retails for $79.95. Each kit contains two product vials and other components. Other stockkeeping units, all cartoned vials, contain a total of four, six, eight, 10, 25, or 50 test strips along with product literature.
Foreign language versions of In Charge are sold in overseas markets including Turkey, Korea, and China. In the United States, In Charge is sold at Wal-Mart pharmacies and other outlets in Texas, New York City, southern California, and Chicago. Additionally, the company accepts catalog and Internet orders.
Vial statistics
The vial that LXN standardized for all test strips is opaque black polypropylene with a UV inhibitor. While the test strips are not overly light sensitive, Hanna says experiments have shown that leaving it in direct sunlight in clear vials for several days can affect test results.
The vials measure 2.5’’ high and have a 1’’ inside diameter. They are received in 2ꯠ-count corrugated cases, each lined with a polybag.
The DesiCap has a 1.3’’ top diameter. Each friction-fit cap comes with a liner of 16-pt clay-coated paperboard that holds a 2-g silica gel desiccant. The caps are supplied in 1ꯠ-count polybags, two bags per corrugated box.
Packaging of In Charge is done in an environmentally controlled 40’ x 60’ room kept at under 30% relative humidity. The glucose test strips are packaged into the vials and capped manually, while the fructosamine strips are automatically produced, collated, filled, and capped in the vials on custom equipment.
Hanna says the products are coded for a one-year “use-by” date printed on the pressure-sensitive label that wraps almost completely around the vial. That’s done off-line to bottled and capped products by a stand-alone Label-Aire (Fullerton, CA) print-and-apply unit started up in ’98. It prints custom information to rollstock that has been preprinted flexo in one or two colors by Pharmaceutic Litho and Label (Chatsworth, CA).
Product cartoning is manual. The tuck-style cartons are 18- or 24-pt SBS paperboard, offset-printed by Royal Paper Box (Montebello, CA) in six colors for the kit and four or five for the other SKUs. The kit contains two vials, one of each type of test strip, smaller vials with control solutions used to verify that the product is functioning correctly, an electronic meter that reads the strips, and product literature.
Alternatives to the DesiCap are few, and cost-effective ones are even more rare.
“Without a desiccant, we’d be looking at some sort of half-million-dollar piece of equipment for the packaging, and we’d probably still need a desiccant even then,” concludes Hanna. That makes LXN’s decision all the more cut and dried.