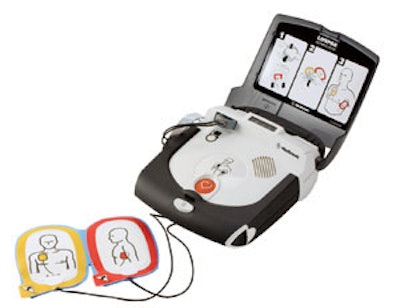
A recent FDA safety alert notified consumers of a voluntary packaging-related recall from Physio-Control. The company is recalling specific production lots of Infant/Child Reduced Energy Defibrillation Electrodes produced by Cardinal Health. Although there are no issues with the performance or function of the electrodes, the artwork on the packaging depicts incorrect electrode placement for an infant. Incorrect placement could result in ineffective energy delivery to the patient and serious injury or death.
Approximately 14,200 electrodes have been affected across four products. The company is notifying consumers of the issue and is providing them with correct electrode placement instructions. Physio-Control is also providing replacement products for all unused affected electrodes.






















