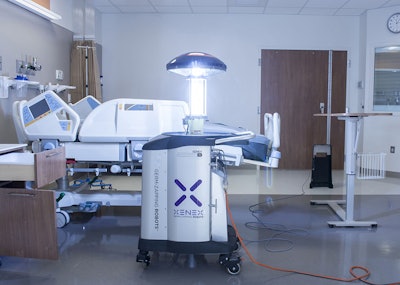
The last thing a patient wants is to develop an infection or catch something new during a stay in the hospital. A recent Popular Mechanics article reported on a new cleanliness trend in hospitals: robots that kill germs. Saint Peter’s University Hospital in New Brunswick, New Jersey has just implemented a LightStrike™ Germ-Zapping Robot™ that uses ultraviolet (UV) light to kill germs in hard-to-clean places.
Xenex developed the robot with new technology that uses pulsed xenon, which is a high-intensity UV light that can penetrate the cell walls of microorganisms such as bacteria, viruses, mold, fungus, and spores. The process fuses the microorganisms’ DNA, rendering them unable to reproduce or mutate. The system is capable of killing dangerous pathogens including influenza, Ebola, and methicillin-resistant Staphylococcus. More than 400 hospitals are using Xenex robots, as well as skilled nursing facilities, ambulatory surgery centers, and long-term acute-care facilities.





















