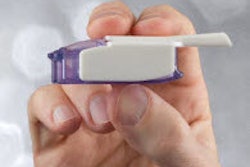
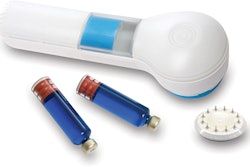
The FDA approved TissuGlu, the first tissue adhesive approved for internal use.
TissuGlu is a urethane-based adhesive that a surgeon can use to connect tissue flaps made during surgery to remove excess fat and skin or to restore weakened or separated abdominal muscles.
Connecting the tissue flaps with an internal adhesive may reduce or eliminate the need for postoperative surgical draining of fluid between the abdominoplasty tissue flaps.
Drops of liquid TissuGluare applied by a surgeon using a hand-held applicator. After applying the drops, the surgeon positions the abdominoplasty flap in place. Water in the patient’s tissue starts a chemical reaction that bonds the flaps together.The surgeon then proceeds with standard closure of the skin using sutures.
“The FDA’s approval of the first synthetic adhesive for internal use will help some abdominoplasty patients get back to their daily routine after surgery more quickly than if surgical drains had been inserted,” said William Maisel, M.D., M.P.H., Deputy Director for Science at FDA’s Center for Devices and Radiological Health.
TissuGlu is manufactured by Cohera Medical, Inc., in Pittsburgh, P.A.