This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
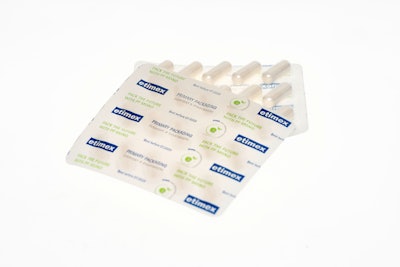
Both components of the Purelay® Pharm blister are made of fully recyclable PP mono, fulfilling the aspects of recyclability that are required of responsible packaging today. As a packaging material, polypropylene provides an excellent barrier against moisture, as well as high transparency and superior wall thickness distribution of the thermoformed tablet blister.
The quality of the high water vapor barrier can be easily checked during the packaging process—it is sufficient to measure the wall thickness distribution. The Purelay® lid component is a push-through plastic film made of filled polypropylene, an innovative alternative to the well-known aluminum push-through films.
Etimex received the German Packaging Award for Sustainability 2021 for the new blister.