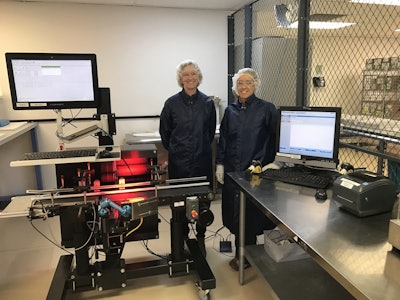
With an international client base that ranges from emerging biotechs to established pharmaceutical giants, it’s no wonder that Afton Scientific®’s Contract Manufacturing Organization (CMO) AnovaFill® is fully compliant with U.S. FDA and European Medicines Agency regulations.
AnovaFill provides cGMP contract sterile filling services of investigative new drugs, biologics, and commercial injectable pharmaceuticals. The facility produces small batch and clinical trial liquid fills of 1 to 100 mL/vial, with production sizes from 100 to 15,000 units and soon to be 50,000 units.
Afton Scientific, AnovaFill’s parent, provides pre-sterilized and custom packaged Ready-to-Fill® components such as sterile vials, stoppers and seals for immediate use in cGMP filling of sterile human drugs. These components are shipped to firms in North America, Europe, Asia, Australia and Africa.
Considered a small contract pharmaceutical facility, AnovaFill works to stay ahead of the competitive curve, particularly when it comes to satisfying 2017 DSCSA serialization deadlines. Much of this “edge” is attributable to its work with partner Videojet. The two companies began working together in late 2015.
According to Ashley Umberger, Afton Scientific’s Manager, Engineering, “FDA guidelines state that the smallest unit placed into interstate commerce by the manufacturer or the repackager that is intended for individual sale should be the item that is serialized. In our instance, we package vials into cartons (in different quantities depending on drug product package), therefore we label cartons as the smallest unit. We implemented both the serialization and aggregation portions now, although aggregation is not required by the November 2017 deadline.”
She states, “When we started our serialization initiative we researched different vendor combinations to get the best in both equipment and flexibility. Videojet designed a system specifically for Afton, giving us an all-in-one-type system.
“We needed a flexible system to accommodate different products in smaller quantities; therefore a rigid, high-volume and elaborate system was not suitable for our needs. Videojet created a system that was flexible, while also catering to other requirements, such as equipment footprint and cost.”
Videojet provided Afton with equipment and related products to print human-readable text and barcodes onto cartons. This includes the coding and control of unit-level information via the Wolke m600 oem thermal inkjet carton printer along with a vision camera system from Cognex and a conveyor system.
Kari Moore, Associate Manager, Quality Assurance, notes the Videojet serialization system prints in black onto primarily white cartons, adding a 2D barcode, product identifier, serial number, lot number and expiration date.
A separate station from Videojet provides a means of aggregating the cartons to a case and cases to a pallet. When aggregating, an operator scans the item and software aggregates each item to create the unique barcode or shipping code (depending on packaging level) needed for that packaging tier, says Umberger
“When the full package quantity is reached, a label will be printed on a Zebra label printer. Scanning of the codes from the label is performed to confirm all encoded information is correct. This label is placed onto the appropriate case and/or pallet and will associate the cases and/or pallets to the unique label code for tracking purposes,” she explains.
Product packaging
Afton’s Anovafil contract manufacturing service provides fully automated aseptic fill-finish in its 20,000 sq-ft manufacturing facilities. All filled products are 100% inspected and vials are automated labeled on equipment from CVC Technologies, then cartoned, serialized and aggregated. Moore points out that Afton primarily uses manual packaging methods due to varying customer needs per product. “Since batches are usually small, we manually box,” says Moore.
Umberger points out that with smaller batch sizes, there are speed and economic advantages to the manual packaging approach. “There are costs associated with larger automated packaging, and there are costs to change out parts or shut down lines for smaller batches.”
Once labeling and packaging are complete, the Videojet equipment comes into play. Moore points out that despite its advanced serialization and aggregation capabilities, the Videojet system allows Afton flexibility to incorporate their current manual process. Items are serialized on the conveyor system and move to the aggregation portion of the line, where operators can manually scan items as they proceed through each packaging tier.
The Videojet system is able to accommodate various product packaging configurations and can adapt to Afton’s dynamic production needs. Moore notes, “The combination of these two methods allows us to be more flexible and we can meet any specifications that our clients require for their packaging needs. For now, we prefer to keep incorporating manual packaging processes for the purposes of speed, space, and the flexibility to quickly accommodate clients.”
Umberger adds, “We can set the packaging line up and take it down whenever and wherever we need to, and the Videojet products lend us that flexibility.
Moore states, “Videojet sent us a system to accommodate all of our coding needs, and that's what was so attractive about this solution. They offered us a complete turnkey package for serialization and aggregation that can serve both facilities. Even though we are a small firm, they treated us like we were a large company. They respected and responded to our ideas.
“We'll definitely get the mileage we need out of our current system. As we gain momentum and start producing more and our batch sizes increase, I can see the lines changing, whether it be increased capacity, additional equipment, or more automation. That’s the direction in which I believe we will head and the Videojet system is scalable and flexible enough to meet our needs for the future” she explains.