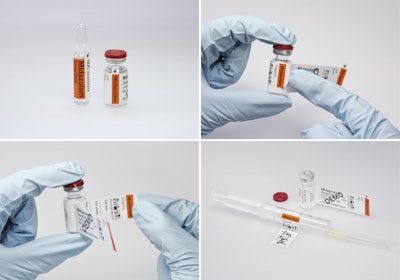
Schreiner MediPharm has developed a new cost-effective label that helps reduce the likelihood of parenteral dispensing errors. The company’s Pharma-Comb Single Wrap (SW) label, a new member of the Pharma-Comb product family, allows a more reliable, simpler syringe dispensing and administration process through a combination of elements that enhance instructional clarity. The label is particularly suited for small containers for single-dose applications, such as ampules and vials.
The supplier notes that each year, an estimated 7,000 people in the U.S. die as a result of inappropriately dispensed drugs. According to the FDA, every third medication error can be attributed to labeling deficiencies. To minimize the risk of dangerous medication errors, the FDA issued a Draft Guidance in April 2013 for the design of pharmaceutical labels and packaging that encourages reliable and simple dispensing. Pharma-Comb SW labels meet these recommendations and assist healthcare professionals in properly dispensing medicines.
Pharma-Comb SW labels provide sufficient space for legally required information, as well as for variable data inscription and barcode printing. Product information is easy to read, and barcode scanning is simple. The reverse side of the detachable section is designed so it can be applied to a syringe in a flag-like fashion that leaves graduation lines uncovered. For greater ease of use when wearing gloves, the detachable part lifts slightly for easy peel-off when opening the label.






















