This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
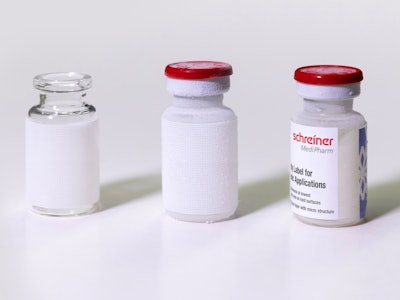
Schreiner MediPharm, a Germany-based global provider of innovative functional label solutions for the healthcare industry, has developed Freeze-Lock, a cryo label that permanently withstands the subzero storage and transport temperatures needed for many cell and gene therapy medications or clinical trials—including those for COVID-19 mRNA vaccines. The new solution comprises two interlocking label layers that ensure reliable adhesive strength and product information readability.
The Schreiner MediPharm Freeze-Lock label consists of two components: a bottom and top label. First, the bottom label layer with a specialty microfine surface texture is applied to the empty, non-refrigerated container at room temperature. Next, the container is filled with the active ingredient and immediately cooled down for storage at subzero temperatures utilizing dry ice (-108.4 °F) or liquid nitrogen (-320.8 °F).
To mark the frozen container, it is removed from cold storage. The Freeze-Lock’s top label layer is dispensed and firmly pressed against the bottom label. (If a layer of ice has formed, this ice does not need to be removed.) The deep freeze adhesive of the top label combines with the bottom label’s texture and freezes in a matter of seconds. The result is a permanent bond between the label construction and the container, which is then returned to storage and transported in frozen condition.
Due to the specialty two-component label design, as well as the materials and adhesives used, the Freeze-Lock cryo label reliably adheres to the frozen container, ensuring important product information is not lost.






















