Pfizer
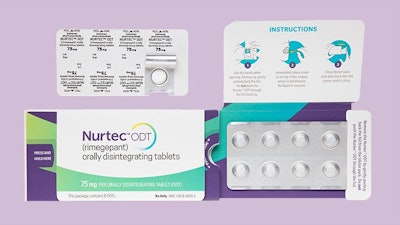
According to a recent FiercePharma article, Pfizer is recalling 4.2 million units of its migraine therapy Nurtec ODT because its blister packaging is not child-resistant. The recall is being carried out in conjunction with the US Consumer Product Safety Commission (CPSC). No injuries have been reported, and a remedy has already been identified. Pfizer has instructed pharmacists to place the implicated blister cards in a child-resistant vial before administering it to patients. The products were sold at pharmacies across the US between December 2021 and March 2023, and were manufactured by Biohaven and Pfizer.