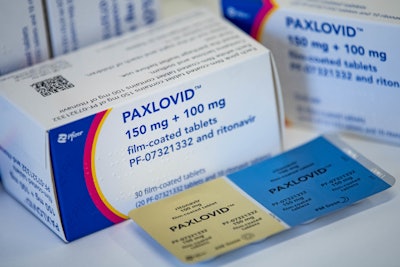
According to a recent Reuter’s article, pharmacists can now prescribe Pfizer’s COVID-19 pill to help improve access to the treatment. Previously, Paxlovid could only be prescribed by physicians and COVID test sites for people at risk of complications from the virus. The FDA has amended the emergency use authorization to make it prescribable by state-licensed pharmacists.