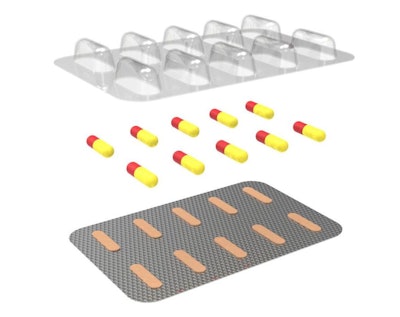
A recent Valdosta Daily Times article noted the approval of a new drug packaging solution intended for oral solid does HIV prevention medicine. The FDA gave the green light to AptarGroup’s Activ-Blister™ packaging that uses silica gel and molecular sieve technology to absorb tailored amounts of water vapor, oxygen, or a combination of the two. It can also scavenge odors and volatile organic compounds.
“I am very pleased to announce this recent FDA approval of our proprietary Activ-Blister ™ technology,” said Stephan Tanda, Aptar’s President and CEO. “This is a significant step that further validates the expanding portfolio of solutions offered by Aptar CSP Technologies. We will continue to leverage our proprietary 3-Phase Activ-Polymer ™ technology to help our customers with unique protective formulations that derisk their drug development process and help strengthen their own offerings. The ultimate result is that we are creating meaningful solutions that help improve and save lives.”