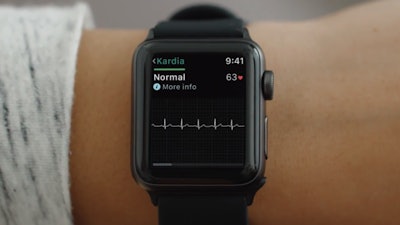
A November 30th Ars Technica article noted the FDA clearance of the first medical accessory designed to work in tandem with the Apple Watch. AliveCor’s KardiaBand parlays the watch’s heart rate technology with a sensor to provide medical-grade EKG readings in as little as 30 seconds. The device looks just like any other band accessory, but contains a silver sensor where users place their finger for a reading. The data can be viewed on the watch’s display in the form of a line graph to inform the user if their heart rate is normal or abnormal.
The device contains artificial intelligence in the form of AliveCor’s proprietary SmartRhythm technology that employs the user’s heart rate history to determine a healthy range relative to activity level. If the watch’s native heart rate monitor detects an abnormality, KardiaBand’s app will prompt the user to take an EKG reading. The device costs just $199, but users must pay the $99 annual subscription to access unlimited cloud storage and history, and customized monthly reports that can be shared with their doctor.