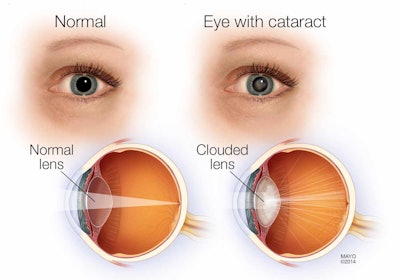
Cataracts are a condition where the lens of the eye becomes clouded and affects vision. The treatment is cataract surgery in which the clouded natural lens is removed and replaced with an artificial one. After the surgery, many patients experience a minor residual refractive error, which means the artificial lens doesn’t focus properly, causing blurred vision. This can be corrected with glasses, contacts, or refractive surgery. However, a recent article from News Medical Life Sciences highlighted a new FDA-approved medical device that provides a better solution.
The Light Adjustable Lens and Light Delivery Device from RxSight Inc. is the first system that allows a physician to make small adjustments to the implanted lens to improve vision after the procedure. The RxSight intraocular lens (IOL) is made from a material designed by a Nobel Prize winning scientist that reacts to UV light delivered by the Light Delivery Device. Patients receive 3-4 light treatments over a period of 1-2 weeks that last about 40-150 seconds. Patients must wear UV protective eyeglasses from the time of the surgery to the end of treatments to protect the new lens. The device is intended for patients without macular diseases who have astigmatism before the cataract procedure.