Antimicrobial-resistant bacteria are becoming increasingly resistant to antibiotics, posing a serious threat to global health. Susceptibility tests that address this threat are expensive, require professional training, and seldom available in remote locations. Researchers at UCLA have developed a simple, inexpensive 3D-printed solution that attaches to a smartphone to perform automated antimicrobial susceptibility testing.
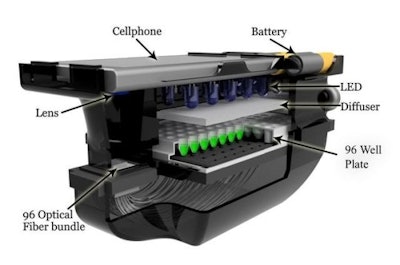
The device uses LEDs to illuminate a 96-well microtiter plate containing a variety of antibiotic doses. The phone’s camera takes photos that measure changes in light transmission in each well. The photos are then sent to either a local or remote server where they’re automatically tested for antimicrobial resistance, and results are sent back to the phone in about a minute. The prototype was developed to work with a Nokia Lumia smartphone, but slight modifications could make it compatible with iOS and Android devices.