How do medical device manufacturers (MDMs) provide double-barrier trays and/or pouches for operating room convenience, yet also provide sustainable packaging? And how do MDMs make packs that readily identify the device inside while are also being easy to read, stack, store, open, dispense, and possibly reuse?
These questions are based on observations gleaned from attending a panel discussion of nurses titled, “Voice of the Consumer,” in which they evaluated generic medical device packages during HealthPack 2009 in Memphis. The annual event returns to the Sheraton Gunter Hotel in San Antonio March 2-4, 2010.
The nurses panel followed a report on the results of a packaging survey of members of the Assn. of periOperative Registered Nurses (AORN). It was the second such survey, building on last year's initial study (see www.healthcare-pack
aging.com/go/39). The goals of the survey are to understand OR user needs, determine what packaging improvements would benefit them, and determine trends and concerns regarding package design and sustainability issues within the hospital environment.
Introducing the AORN presentation was Jennifer Neid of Oliver-Tolas Healthcare Packaging. Neid also serves on the Institute of Packaging Professionals' Medical Device Packaging Technical Committee. Survey results were reported by Jennifer Blocher, then a Masters Degree candidate in Packaging Science at Clemson University. Clemson, IoPP, and industry members were involved in the survey. More than 100 professionals responded. Among the findings were the following:
• The majority of respondents (57.7%) were in the 51 to 60 age group, 90.9% were female, and 53.2% indicated they had at least 31 years of medical experience. The aging nursing demographic means that some pack openings are too small, especially for nurses developing arthritis and related ailments. Vision challenges also play a role, with smaller print font sizes proving more vexing.
• Speed of opening the package was ranked as the most important feature with respect to medical device packaging, followed by producing the least amount of packaging waste, offering the smallest possible package, and dealing with consistent packaging sizes from MDMs.
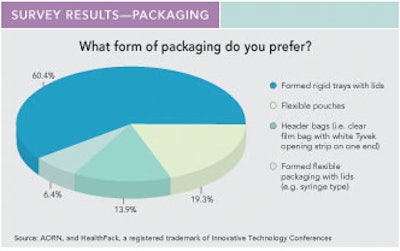
• 82% of respondents preferred double-entry packaging for greater sterility, safer handling, and less risk of contamination. Respondents cited ease of presentation, easier dumping/flipping, room for error if the package is dropped, and device sterility as important attributes for double-entry device packaging.
• Header-bag opening fixtures, corner-peel opening of a peel pouch, and packaging material tearing were the most frequently identified packaging characteristics that have caused challenges for survey respondents. One respondent said, “Frequently, there is not a good way to grasp the packaging, or the ends are almost fused together and you have to try to find an area that allows separation and peel back.” Another respondent was especially troubled by Chevron-style packs, saying “Now there is nothing [to grab onto], and it is a struggle to open these types of packages. The industry has gone on the 'cheap' from this aspect.”
• Labeling-related issues remained a critical medical device packaging concern. Nurses voiced frustrations in finding and being able to read use-before dates, instructional details, and color-coding. Color-coding from various MDMs makes it difficult for nurses, particularly when one specific color has different meanings for different MDM packages. Nurses may be trained NOT to recognize colors to avoid potential problems. Sterilization indicator tags or labels were challenging for nurses to locate on the pack.
• Identification of device manufacturer, model number, and use-before date were listed as the most important labeling aspects. Next were easily-read text/font, followed by color-coded labeling, manufacturer's instructions for use in every package, and manufacturer's instruction for use provided via the Web or a CD.
• Devices were most frequently rejected due to dirt, hair, or foreign materials, for damaged packaging, or due to materials that tore or shed fiber during opening.
• 12.5% of respondents said they've used a device where sterility was questionable, perhaps as a result of dropping the package and reusing it after visually inspecting the pack. If a circulating nurse drops the sterile package before presenting it to the sterile nurse, 37% of respondents said they inspect the pack, 30.6% said they would present the device from the inner package, 27.8% said they would discard it, and 4.2% opted to re-sterilize the pack.
• Despite the concern for smaller packages using less material, 90% of respondents noted used packaging materials are disposed into the trash; 87% of respondents said their facility does not recycle materials; and 65.2% indicated that recycling is impossible because there's no place to recycle the used materials.
These questions are based on observations gleaned from attending a panel discussion of nurses titled, “Voice of the Consumer,” in which they evaluated generic medical device packages during HealthPack 2009 in Memphis. The annual event returns to the Sheraton Gunter Hotel in San Antonio March 2-4, 2010.
The nurses panel followed a report on the results of a packaging survey of members of the Assn. of periOperative Registered Nurses (AORN). It was the second such survey, building on last year's initial study (see www.healthcare-pack
aging.com/go/39). The goals of the survey are to understand OR user needs, determine what packaging improvements would benefit them, and determine trends and concerns regarding package design and sustainability issues within the hospital environment.
Introducing the AORN presentation was Jennifer Neid of Oliver-Tolas Healthcare Packaging. Neid also serves on the Institute of Packaging Professionals' Medical Device Packaging Technical Committee. Survey results were reported by Jennifer Blocher, then a Masters Degree candidate in Packaging Science at Clemson University. Clemson, IoPP, and industry members were involved in the survey. More than 100 professionals responded. Among the findings were the following:
• The majority of respondents (57.7%) were in the 51 to 60 age group, 90.9% were female, and 53.2% indicated they had at least 31 years of medical experience. The aging nursing demographic means that some pack openings are too small, especially for nurses developing arthritis and related ailments. Vision challenges also play a role, with smaller print font sizes proving more vexing.
• Speed of opening the package was ranked as the most important feature with respect to medical device packaging, followed by producing the least amount of packaging waste, offering the smallest possible package, and dealing with consistent packaging sizes from MDMs.
• 82% of respondents preferred double-entry packaging for greater sterility, safer handling, and less risk of contamination. Respondents cited ease of presentation, easier dumping/flipping, room for error if the package is dropped, and device sterility as important attributes for double-entry device packaging.
• Header-bag opening fixtures, corner-peel opening of a peel pouch, and packaging material tearing were the most frequently identified packaging characteristics that have caused challenges for survey respondents. One respondent said, “Frequently, there is not a good way to grasp the packaging, or the ends are almost fused together and you have to try to find an area that allows separation and peel back.” Another respondent was especially troubled by Chevron-style packs, saying “Now there is nothing [to grab onto], and it is a struggle to open these types of packages. The industry has gone on the 'cheap' from this aspect.”
• Labeling-related issues remained a critical medical device packaging concern. Nurses voiced frustrations in finding and being able to read use-before dates, instructional details, and color-coding. Color-coding from various MDMs makes it difficult for nurses, particularly when one specific color has different meanings for different MDM packages. Nurses may be trained NOT to recognize colors to avoid potential problems. Sterilization indicator tags or labels were challenging for nurses to locate on the pack.
• Identification of device manufacturer, model number, and use-before date were listed as the most important labeling aspects. Next were easily-read text/font, followed by color-coded labeling, manufacturer's instructions for use in every package, and manufacturer's instruction for use provided via the Web or a CD.
• Devices were most frequently rejected due to dirt, hair, or foreign materials, for damaged packaging, or due to materials that tore or shed fiber during opening.
• 12.5% of respondents said they've used a device where sterility was questionable, perhaps as a result of dropping the package and reusing it after visually inspecting the pack. If a circulating nurse drops the sterile package before presenting it to the sterile nurse, 37% of respondents said they inspect the pack, 30.6% said they would present the device from the inner package, 27.8% said they would discard it, and 4.2% opted to re-sterilize the pack.
• Despite the concern for smaller packages using less material, 90% of respondents noted used packaging materials are disposed into the trash; 87% of respondents said their facility does not recycle materials; and 65.2% indicated that recycling is impossible because there's no place to recycle the used materials.