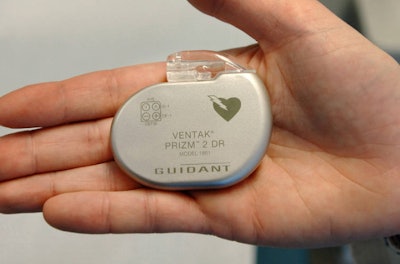
A Wall Street Journal article dated July 15th discussed Medicare’s recent endorsement of unique identification numbers in billing records for medical devices. Despite years of persuasion from lawmakers and the FDA, this is the first positive acknowledgement of ID numbers by the Medicare agency.
Should the change occur, ID numbers would be stored in databases, allowing the FDA to easily monitor malfunctions and issue recalls accordingly. The article cites a specific heart defibrillator that erroneously shocked patients. While physicians were aware of the malfunction, it took roughly ten months for the company and the FDA to pull it from the market. By that time, 268,000 defective devices had been implanted. If the company had access to pivotal evidence of the malfunctions provided by ID numbers, the situation wouldn't have escalated to that unfortunate magnitude.