Orthofix is a global medical device company focused on developing products for the spine and orthopedic markets. When developing its FORZA™ spinal medical devices, Orthofix sought to avoid creating a separate package for each of the approximately 700 different size spinal devices.
To minimize packaging components, the Lewisville, TX-based company worked with its supplier Placon, a manufacturer of stock and custom thermoformed packaging, and Placon’s medical packaging division, Barger.
Dewey Waites, Sterilization and Packaging Engineer for Orthofix Spinal Implants, was involved in the FORZA development project. “FORZA is a trade name for one of our spinal implants used in surgical procedures and packaged here at our Lewisville facility,” says Waites. “Orthofix packages four primary spinal implant product lines made with PEEK, a polyetheretherketone thermal rigid material and with PEEK material molded in between two titanium plates used for Cervical Interbody fusion.
“Within these four different product lines there are about 700 total SKUs in different sizes and configurations,” says Waites. “I think what sets our packaging apart from other companies is our universal package that accommodates all of the spinal implants. That’s the key.”
Trays, lidding, and retainers
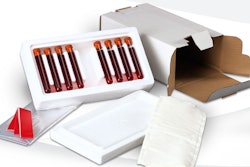
The FORZA packaging project, developed jointly by Orthofix and Barger, consists of a total of four thermoformed PETG trays; one inner tray, two sizes of inner retainer trays, and an outer tray. The inner tray houses a retainer that holds the actual implant. Both the inner and outer tray are sealed with coated DuPont™ 1073B Tyvek® lidding material.
The PETG retainer housed within the inner tray of the double-sterile barrier tray system comes in two sizes. One is designed to hold “small” and “medium-sized” spinal implants, while a second retainer is manufactured to accommodate “large” implants.
Mike Nielsen, national sales manager, Barger, says, “Barger was able to take “worst-case” sizes, or the smallest and largest implants into account while ultimately designing for spinal parts with the largest volumes. To create cost-effective packaging that is user-friendly, two different-sized retainers were developed that can hold all small, medium, and large implants. By dual-purposing the small/medium-sized retainer, Barger was able to reduce the number of components needed, consolidating the need for three different retainers to two by utilizing the same thermoform for the small and medium-sized implants. This in turn lowered the amount of tooling costs as well as the number of packaging part SKUs Orthofix needs to inventory and manage.”
“Utilizing unique mold and die designs allowed moving the die-cut flange to a mid-height position in the dual-purpose small/medium retainer combo,” states Kelly South, Barger’s lead design engineer for the Orthofix project. “Not only did this allow the retainer to house two separate size groups of implants (A and B), but the mid-height die-cut flange also serves for self-locating the retainer within the inner tray, further reducing movement of the implants within the packaging. The large retainer also utilizes the same mid-height die-cut flange, again reducing the ability of the implants to move within the thermoform.”
Packaging process
Within a Class 7 ISO certified cleanroom at Orthofix, the implants are manually placed inside the retainer tray that is then placed within an inner tray and heat-sealed with the die-cut Tyvek lidding material. This is done on a heat-sealing machine, six trays at a time. That sealed tray is then placed within a slightly larger tray and again sealed with Tyvek lidding on the same double-shuttle seal machine using an inner and outer seal tooling.
Following sealing, double-barrier trays are placed into a folding carton, supplied by Barger. A pressure-sensitive label is placed on the outer tray’s lid and the folding carton includes a product schematic of the implant, description, lot number, quantity, and barcode information. Orthofix places Instructions for use and record labels into the carton containing the double-barrier tray.
The folding carton is then shrink-wrapped, placed into an outer shipping case and sent to an outside firm for gamma irradiation sterilization. Once sterilization is completed, the implants are returned to Orthofix for ultimate distribution to hospitals and clinics. Waites says the double-barrier tray is preferred by operating room staff.
As for the tray materials, Waites says the important characteristic is that the barrier is not damaged and the product remains sterile. “Both the outer and inner trays are opened within a sterile field,” adds Waites. “The PETG material is tinted light blue, so it’s very clear, allowing users to visually identify the product inside the package.
“It is a universal package for us. Instead of having packages for each of the 700 parts, the package design allows all of those products to fit into four package sizes. That reduces inventory space here at our facility, and at the hospital, where space is also limited.”
Looking back at the development process, Waites notes, “In going with a universal package, there were multiple design validation studies performed on the packaging prior to releasing it to production. That’s the key for us because we wanted to make sure the integrity of the package is maintained. Once you ship the product you may never know what happens to it when it leaves the plant. Upfront design validation is important and following ASTM and ISO design and test standards and procedures, to ensure package integrity.”