For decades, the go-to treatment for attention-deficit/hyperactivity disorder (ADHD) has been a prescription for amphetamine (Adderall) or methylphenidate (Ritalin). However, a new prescription-only medical device, and first non-drug treatment, could soon be on the market to compete. According to a recent USA Today article, the Monarch external Trigeminal Nerve Stimulation (eTNS) System was just cleared for marketing by the FDA.
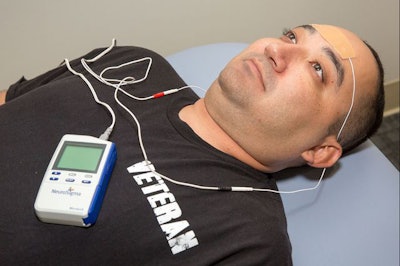
The Monarch eTNS System is intended for children ages 7 to 12 who are not taking ADHD prescription medication. A wire connects the device to a small patch on the forehead of the patient and generates a low-level electrical pulse to the trigeminal nerve, which relays a therapeutic signal to the part of the brain that regulates attention, emotion and behavior. Clinical trials suggest it can take up to four weeks for progress to become evident.