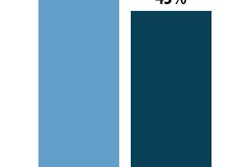
From the Marchesini Group, MA 400 guarantees extremely fast changeover times and low maintenance. It is compact—without compromising the machine’s high-speed output—and is easy to use, thanks to a range of control functions that guarantee maximum efficiency, and a new operator interface called “Easy Door.”
This version has several improvements from both a functional and aesthetic point of view. The new software makes the operating system more powerful and faster. The wider screen is more ergonomic and sensitive, similar to that of a mobile device.
MA 400 is suitable for high-speed packing of blisters, bottles and vials, rigid and squeezable tubes, sachets and trays for pharmaceutical and cosmetic markets.