This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
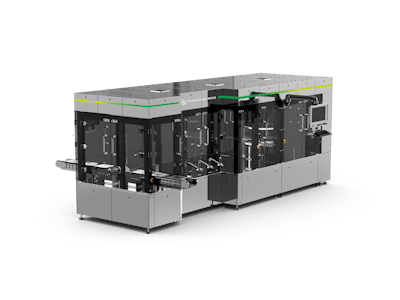
This week at interpack, Stevanato Group presented its latest advances in automatic visual inspection, assembly, and packaging equipment for the pharmaceutical industry, such as MAVIS Combi, a state-of-the-art machine for automatic visual inspection.
MAVIS Combi:
- Is suitable for any glass-filled drug container solution (syringes, vials, ampules, cartridges)
- Inspects liquids, suspensions, gel-like and lyophilized substances
- Performs advanced visual inspection with a small footprint
- Achieves speeds up to 400 pieces/hr
- Offers ease of use, installation, and maintenance
- Provides enhanced safety and accuracy, costs reduction, optimized production timing
Stevanato Group also showcased a live demo of a high-speed auto-injector assembly line, designed to meet the market requirements for flexibility, efficiency, and scalability to streamline drug delivery device programs, at Interpack Booth #17A73.
























