When we hear success stories, we often look for patterns that we can apply in our own lives. Serialization in pharmaceutical packaging is no exception. During a presentation at the Healthcare Packaging & Processing Conference co-located with this year’s PACK EXPO East in Philadelphia, Glenn Siegele, President and CEO at Omega Design Corporation, explained that serialization success stories share common themes. Each story has a clearly defined process strategy and a path to implementation. The underlining message in this presentation was to emphasize that serialization solutions may be unique to each application, but there are common approaches to solving them.
Process strategy
Siegele presented four typical process strategies that pharma companies can take:
-
Serialize each of their product lines
-
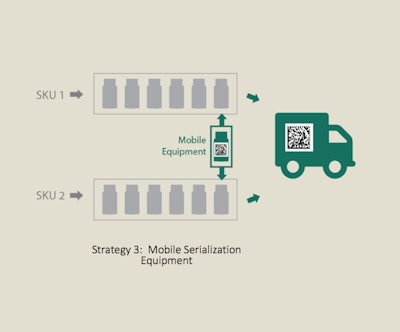
Consolidate product (SKU) into fewer lines that get serialized
-
Move serialization equipment between lines
-
Move product to a serialized work center(s)
Each strategy introduces pros and cons. It’s the pharma company’s role to prioritize which factors are most important:
-
Total Cost of Ownership (TCO), which includes upfront capital costs, line down time, impact on line efficiency (OEE), implementation and support costs
-
Work In Progress (WIP) Management
-
Serialization Capacity and Percent Utilization
-
Production Scheduling Flexibility
-
Production Redundancy (risk management)
Implementation path
Once you determine which lines will be serialized, you will need to choose a path to implement equipment onto those lines.
Depending on your needs, the implementation path will be either a custom or pre-configured system. In this stage, you have to determine your priorities in terms of cost, complexity, features, the level of your design input, and delivery time. Of course, a custom system will come at a higher cost and longer lead time, but will offer more flexibility for features, complexity and your specific design requirements.
Siegele presented three real world examples to highlight the process strategies and implementation paths involved.
Success story #1: a custom application for vials
With this very complex application, Omega worked with a large company that needed to serialize small glass vials and plastic bottles for their Chinese animal health division. Only a small segment of their product (2%) was getting serialized and the company was concerned about line disruption with the other 98%. The other major challenge was managing product from other sites within the company that they were planning to route to this line for serialization.
Additional challenges included a complex physical layout where space was at a premium, multiple packaging formats (bottles, vials and cartons), glass vials and UV coding, and Environmental, Health and Safety (EHS) concerns.
In this case, the company’s preferred process strategy was to move product to an offline serialized work center. Here, products would travel from primary labeling to a system that codes the caps and associates the cap code with the label. Then the product would move to manual carton loading, carton coding and finally to case aggregation. The company chose to implement custom equipment due to the project complexity and the customer’s need for design input.
Success story #2: rapid deployment for cartons
A contract packager approached Omega when they needed a case aggregation system for a dedicated serialization line, where an OEM was packaging injectable pens inside rectangular cartons. The packager was flexible with code placement and code size, but needed to perform the FAT for one carton configuration as soon as possible.
After examining utilities, vendors, case label size, and vision requirements, Omega found that a pre-configured, rapid deployment system using the strategy of serializing each line was a good fit for the cartoning operations, where design and delivery time were highest priority.
A semi-automatic case packing system was selected. The system was integrated with a pre-tested serialized data management system, and Omega provided a standard Detailed Design Specification (DDS), and validation support. The FAT took place only eight weeks after the order was received.
Success story #3: mobile serialization cell
When a major pharmaceutical company needed to code cartons and aggregate cartons-to-cases, they knew they wanted the capability and flexibility to bring end-of-line serialization anywhere within the plant. They were incorporating three different strategies within their plant: (1) converting a full-capacity line into a high-speed, fully automatic serialization line, (2) creating another serialized line that consolidated multiple SKUs, and (3) using mobile serialization equipment to provide serialization for four other lines.
Process Strategy #3, which involves moving serialization equipment between lines, fit the company’s needs for flexibility. They selected the Intelli-Code Carton Coding & Inspection System and Intelli-Pac Semi-Automatic Serialized Case Aggregation System.
The equipment was designed for mobility, with all components on a single frame, an easily integrated handle option, and portable conveyors with quick-disconnects. Conveyors and electrical connections can be separated, repositioned and reconnected, allowing for changeover in under 10 minutes.
As always, there is no one-size-fits-all solution when it comes to serialization. But you can choose a strategy and implementation plan that fits your line. As Siegele explained, “Let your needs dictate your path to implementation.”