This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
IL Group, a specialist in multifunctional labeling solutions for the pharmaceuticals and healthcare sectors, played a critical role in helping ExelaPharma Sciences—a U.S.-based specialty pharmaceutical company and contract development & manufacturing organization (CDMO)—launch a series of prefilled syringe products requiring tailored tamper-evident solutions. The partnership eliminated a hurdle in Exela’s go-to-market timeline, providing a custom-designed label that addresses regulatory and safety requirements, but is also compatible with the company’s existing production processes.
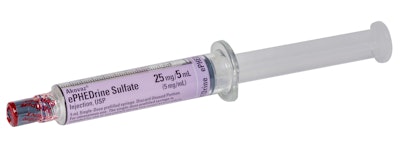
The challenge arose as Exela prepared to introduce several products in plastic cyclic olefin copolymer (COC) syringes. These syringes did not include a manufacturer pre-installed tamper evident feature—a safety element for regulatory approval and patient assurance. After exploring several domestic supplier options without success, Exela turned to IL Group, a pharmaceutical labeling manufacturer with global reach offering relevant shrink film label solutions.
Drawing on its portfolio of proprietary shrink label materials and in-house design expertise, IL Group developed a custom label compatible with Exela’s current labeling operations, avoiding the need for costly and time-consuming equipment modifications. Featuring a tamper-evident seal tailored to the syringe format, the design was delivered on a tight turnaround to help keep the product launch on schedule.
“Our collaboration with IL Group solved a critical packaging challenge in a condensed timeframe,” says Phanesh Koneru, Ph.D., J.D., LL.M., President and CEO of Exela Pharma Sciences. “They provided not just materials, but true design support—something we could not find elsewhere in the U.S. market.”
Since the launches, Exela has been able to meet growing demand for therapies delivered via prefilled syringes—products that require robust safety features to ensure both supply chain security and patient health. The collaboration with IL Group was instrumental in delivering these products to market with the integrated protections today’s healthcare environment demands.






















