This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
Schreiner MediPharm, a Germany-based global provider of functional label solutions for the healthcare industry, and Plas-Tech Engineering, an expert in prefillable polymer syringes, have entered into a collaborative partnership for Cap-Lock – an innovative first-opening indication for prefilled syringes.
Schreiner MediPharm initially launched the Cap-Lock cap adapter in the fall of 2019 and, less than a year later, introduced a product evolution featuring a label-integrated inlay for automated supply chain management and digital first-opening indication. Now, the development partnership between Schreiner MediPharm and Plas-Tech Engineering optimizes industrial manufacturing of the cap adapter, providing pharmaceutical manufacturers with a full-service solution for equipping luer-lock syringes with reliable tamper evidence.
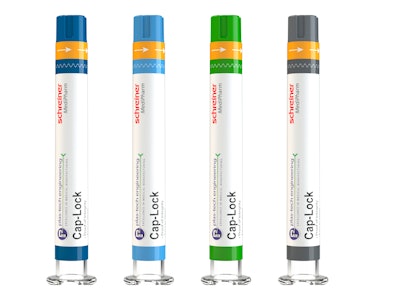
Cap-Lock is a security concept for prefilled syringes developed by Schreiner MediPharm combining a cap adapter and label. Cap-Lock irreversibly indicates whether a syringe has been previously opened, ensuring the integrity of the primary container and preventing its undetected opening. Unlike shrink-wrap solutions, Cap-Lock is also suitable for heat-sensitive substances.
Specializing in customized prefillable polymer syringes and injection-molded parts, Plas-Tech Engineering develops and produces the cap adapters. The cap is precisely adapted to the syringe, and ensures accurate interlinking with the primary closure to equalize the diameter differences between the syringe body and closure. The cap adapter can be produced in a variety of colors per desired branding. In addition, customization of the cap surface texture is available to support safety and ease of syringe opening. Plas-Tech Engineering can deliver the syringe plus applied cap as a validated system.
Schreiner MediPharm designs the tamper-evident marking label to suit customer- and application-specific requirements. The label can be provided with additional functionalities such as counterfeit-protection features, detachable documentation labels, light protection, and an integrated RFID chip. Thanks to Cap-Lock’s construction, the label can be applied to the syringe via conventional labeling processes.