Noble, a global provider of medical device training, patient onboarding strategies, and multisensory product development for the world’s top pharmaceutical brands, recently unveiled a new product platform for safety demonstration devices.
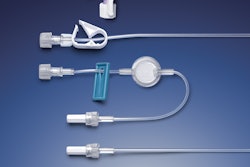
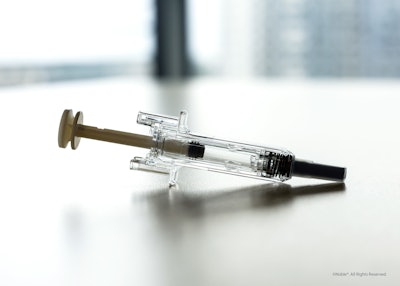
Noble’s new product platform replicates the user experience of the BD UltraSafe Plus™ and BD UltraSafe Passive™ needle guard portfolios and incorporates core device features and capabilities based on BD UltraSafe™ 1 mL and 2.25 mL needle shielding technologies.
Recognizing that proper onboarding is an essential component for patient compliance and safety when starting a self-injection treatment regimen, Noble launched a new product platform for prefilled syringe (PFS) demonstration devices (trainers) that replicate the user experience of BD (Becton, Dickinson and Company)'s UltraSafe™ Needle Guard portfolio.
BD's pharmaceutical partners now have the option to provide a multi-sensory training device, customized for each product, that can enhance the patient training experience by closely replicating the PFS.
The platform now incorporates an extensively tested, proprietary lockout mechanism. Designed for repeated use, this resettable locking needle guard simulates the lockout forces of BD safety and shielding systems for prefillable syringes (PFS), with the capability for patients to reset the mechanism for multiple refresher sessions prior to actually injecting.
Through extensive research, Noble developed demonstration device specifications that best replicate the injection experience. That enabled the company to tool a product platform, which allows for speed-to-market and fixed production costs.
Companies that utilize the BD UltraSafe Plus and BD UltraSafe Passive needle guard portfolios for their drug delivery can now benefit from the demonstration device platform.
Additional specific features of the Noble product platform include:
• Needle tip simulation options: Realistic injection simulation, with encased faux needle tip, designed to simulate the feel and forces involved with an injection.
• Consistent training experience: The platform mirrors the design, form, and function of a PFS with safety and shielding systems, simulating all aspects of the patient experience while offering a consistent and repeatable injection training experience through a defined reset position.
• Customizations: Flanges and plungers are available in both standard and client-customizable configurations, and both rigid and soft needle shield options are available.
(See related story on Noble’s partnership with BD to train patients to use advanced drug delivery devices.”