In a nutshell, FDA law depends on the product.
The best first step in any analysis is to establish what type of product you are dealing with. That's simply because drugs, medical devices, biological products, foods, and cosmetics are each regulated via different requirements about how they are cleared for legal marketing, manufactured, and labeled, among other requirements. So to know what game you're in, you have to know the type of product.
And then along come “combination products” just to throw everyone off base. The increasingly important field of individual products that combine, say, medical devices and drugs or biological products (blood products, vaccines) is as full of promise and exciting medical benefits as it is challenging regulatory questions.
For those who prefer the definition in the regulation, it says that combination products include, “A product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are physically, chemically, or otherwise combined or mixed and produced as a single entity.” But it also covers separate products packaged together “in a single package or as a unit.” The key concept is that all the component products are required in order to achieve the intended use, indication, or effect.
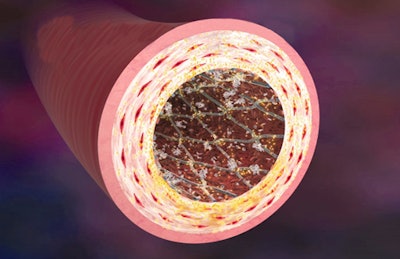
As an example, think of a drug-coated stent, an early and classic combination product. The stent is a piece of metal inserted into a blood vessel to physically hold it open. But if you slather it with a drug then it will drizzle the drug into the area as well as hold the vessel open, thereby providing both a device effect and a drug effect.
Talk to Michael Drues, PhD, about combination products, which he has worked on for 20 years or so, and you start to appreciate both the promise and the roadblocks. Dr. Drues is president of Vascular Sciences of Grafton, MA, a consultant to drug, device, and biological product companies on product development and design, technology assessment, and regulatory affairs, and he also consults regularly with FDA.
Drues, who will discuss “New Developments in Combination Products and Working with the FDA” at the May 26 Healthcare Packaging Conference and Workshop 2011 in Princeton, NJ, thinks FDA's approach of assigning a lead center according to a product's primary mode of action is actually a problem. “We're trying to take these new technologies and spin them to fit into one of these old 'silos,'” he says, instead of finding ways to modify the regulatory model. When he calls those traditional product definitions “silos,” he means narrow categories with boundaries.
Instead, he says amend the law and regulations to tailor a regulatory pathway for combinations, rather than trying to force them into the old categories, and that will encourage them to be developed, he says. Right now, the uncertainty can discourage development.
“It's a huge problem,” he says. He makes the analogy to the intellectual property field: If you have a hot new product but the path forward is unclear as to whether you can patent it, you might decide not to pursue it.
In the same way, Drues says, some really exciting and promising medical technologies are being thwarted before they begin, simply because it's not clear to the companies involved how the technologies would be regulated, what they'd have to prove to get them approved, and so on.
Food packaging companies may remember a similar pattern before the Food Contact Notification program was put in place. Packaging makers were reluctant to develop any product that would require a Food Additive Petition due largely to the uncertain timing of such petitions. By contrast, the FCN program has a predictable 120-day review time (and exclusivity for the notifier, to boot).
Intended use
If figuring out what kind of product you have is key, the crux of doing that is, in turn, determining the product's intended use. Combination products have an intended use that encompasses both or all of their component products. That is, the manufacturer intends that they be used together to achieve an effect, either therapeutic or diagnostic, that the individual products would not achieve separately.
Packaging challenges abound when you create some of these combinations. A metal article may be easy to package and sterilize, but not so easy when it's infused with a drug chemical or biological substance that can't hold up to the sterilizing treatment. Shelf life might be easy to maintain in a packaged, inert metal or plastic device, but not so easy when it contains a drug or biological substance. It's not yet clear what will be the packaging solutions to some of these vexing problems.
The first issue FDA asks itself when confronted with a new drug-device-biologic combination is, 'Which of our departments will regulate it, the drug folks at the Center for Drug Evaluation and Research, or the device folks at the Center for Devices and Radiological Health, or the biological folks at the Center for Biologics Evaluation and Research?'
They figure out what they think is the component product that provides the overall product's “primary mode of action,” and assign the combination product to that agency center.
Exciting future possibilities
Drues can point to some truly Brave New World possibilities on the horizon. He describes an ultimate combination of a drug, device, and biological product, referred to as tissue engineering. You'd combine growing tissues, which are a biological product; a housing or tray to hold it during growth, which is a device; and the tissue creates a therapeutic protein or other chemical, a drug.
Looking ahead, more category or “silo” challenges can be expected. Drues thinks devices to deliver dietary supplements with beneficial health effects, or even traditional foods, could be in our future.
And it won't just be the regulators who will be facing challenges to know how to address these new types of products—also packagers. He says, with disarming understatement and a pun, “These problems will force them to think outside the box.” Or the silo.
-Eric F. Greenberg, Attorney-at-Law, Eric F. Greenberg, PC.
In the photo, drug-eluting stents are coated with medication (indicated with yellow dots) to reduce the chance of an artery becoming clogged again by slowing the growth of excessive tissue as the artery walls heal following angioplasty. The medication is released, or eluted, into the arterial wall over a period of a few weeks. Photo courtesy of Medtronic, Inc.





















