Los Angeles-based Grifols USA, LLC is a global corporation with manufacturing facilities in that city as well as in Barcelona, Spain, where the company was founded in 1940. It specializes in the production of blood and plasma therapies to treat various life-threatening diseases and conditions.
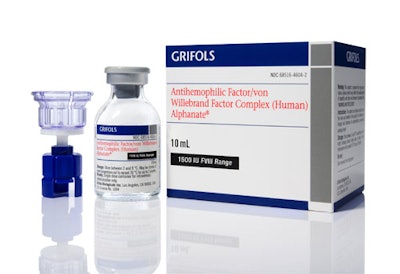
One of those therapies, AlphaNine® SD Coagulation Factor IX (see photo) is for the prevention and control of bleeding in patients with Factor IX deficiency due to hemophilia B.
“We continually strive to meet our customer's needs and enhance the convenience and ease of using our medicines,” says Bill Stopher, president of Grifols USA. With that in mind, the company recently announced the availability of West's () Mix2Vial filtered transfer system for the reconstitution of Grifols' coagulation therapies.
Developed and manufactured by West's subsidiary Medimop Medical Projects Ltd., Mix2Vial is a patented device designed for easy reconstitution of lyophilized drugs. The plastic, needle-free device is manufactured with a built-in 15-micron filter that's designed specifically to be used with the sterile water for injection diluent packaged with Grifols' coagulation products.
“Introduction of the Mix2Vial transfer set represents just one more step we can take to address the challenges of living with hemophilia,” notes Stopher.
Watch an in-action visual of Mix2Vial here.
One of those therapies, AlphaNine® SD Coagulation Factor IX (see photo) is for the prevention and control of bleeding in patients with Factor IX deficiency due to hemophilia B.
“We continually strive to meet our customer's needs and enhance the convenience and ease of using our medicines,” says Bill Stopher, president of Grifols USA. With that in mind, the company recently announced the availability of West's () Mix2Vial filtered transfer system for the reconstitution of Grifols' coagulation therapies.
Developed and manufactured by West's subsidiary Medimop Medical Projects Ltd., Mix2Vial is a patented device designed for easy reconstitution of lyophilized drugs. The plastic, needle-free device is manufactured with a built-in 15-micron filter that's designed specifically to be used with the sterile water for injection diluent packaged with Grifols' coagulation products.
“Introduction of the Mix2Vial transfer set represents just one more step we can take to address the challenges of living with hemophilia,” notes Stopher.
Watch an in-action visual of Mix2Vial here.






















