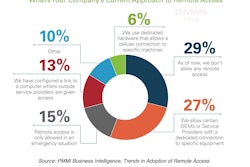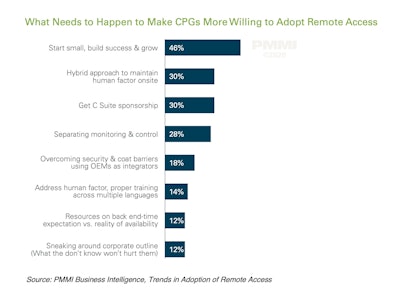
This was among the findings in a recent report from PMMI Business Intelligence called Trends in Adoption of Remote Access. As the chart included here shows, it may also be helpful to implement a hybrid approach to remote access that involves maintaining a human factor onsite. And let’s not forget about the importance of obtaining C-Suite sponsorship from day one, not to mention winning corporate support. And one good way to win that support, the report points out, is to look initially at the first level of management, the change agents, and then build the decision around a strategic pillar to the corporate level. Other solutions touched on in the report include these:
• CPG IT departments should be included at the beginning of projects to increase IT’s understanding of how remote access at the OT level works.
• OEMs need to ensure better documentation of previous technical service so as to ensure continuity.