This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
Cetec ERP, a full-suite provider of web-native ERP systems for manufacturers, releases versions 4.19 and 4.20 designed with new capabilities to support the day-to-day compliance and quality needs of medical device and life science companies. The updates deliver greater control over regulated processes, quality tracking, and supplier oversight—all within a single integrated ERP system.
Customizable Device History Record Generation
One of the features is a customizable Device History Record (DHR) document that is automatically generated and tied to the order, work order, and invoice. This consolidated record captures the complete manufacturing history for each device, making it easier for manufacturers to prepare for audits and meet documentation expectations under FDA Quality System Regulation and ISO 13485.
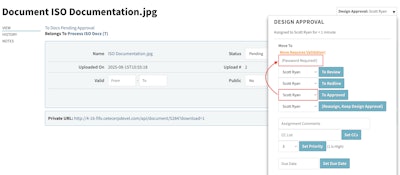
Password Validation for Workflow Approvals
A new “Require Validation” option in workflow stages prompts password reentry before an object can advance. For companies operating in regulated environments, this adds an important safeguard for electronic authorizations and supports secure sign-off requirements often associated with FDA 21 CFR Part 11.
More Flexible CAPA Tracking
The Corrective Action Report (CAR) module now supports user-defined fields, allowing quality teams to capture additional details during investigations and follow-up. This added flexibility strengthens Corrective and Preventive Action (CAPA) programs by making it easier to track, analyze, and retain the information that matters most for compliance and quality improvement.
Quality Performance at a Glance
New home page dashboard widgets give real-time visibility into inspection results. A pie chart displays inspection failure codes while an “Inspected vs. Rejected” widget shows quantity metrics, yield percentages, and PPM. These tools help quality managers identify trends and address issues before they escalate.
Supplier and Production Record Enhancements
“For manufacturers in regulated industries, compliance and quality are constant priorities,” says Taylor Wagen, COO at Cetec ERP. “These updates are about giving teams more control and clarity over their processes, so compliance isn’t a separate burden—it’s simply part of how they run their business in Cetec ERP.”