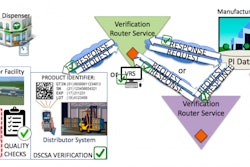
QU4RTET, whose sotfware license is owned by Serial Lab, is the first open source platform for level 4 serialization, designed to give pharmaceutical companies freedom, transparency and security in meeting the requirements of the federal Drug Supply Chain Security Act (DSCSA).
QU4RTET helps manufacturers by generating and managing unique product identifiers for printing on saleable units of a prescription drug, from bottle to carton to pallet to case. The platform orchestrates level 4 serialization—communication between an organization's business systems and manufacturing/packaging lines. It supports current GS-1 communication standards, both EPCIS 1.1 and 1.2. It also an option for repackagers, as it supports transformation event processing where inbound product is assigned new serial numbers. All of the relationships from the original serial numbers are maintained in the system.
User flexibility
Since QU4RTET is open source, customers do not pay software license or transaction fees or wait for needed features to become available. As an open source product, an organization can create, fund and contribute a specific feature at any time. The company notes, for example, that a developer working for a pharmaceutical manufacturer in Russia might decide to add Russian serialization features over and on top of existing master-material and EPCIS features in the product.
Vantage Solutions, provider of manufacturing productivity services,has funded the initial development and is dedicated (along with a few other companies) to add regulatory features as they are needed. Vantage offers validation services, installation and consulting around QU4RTET, but the company released the source code to the public domain and he system can be used and installed by anyone without the help of Vantage.