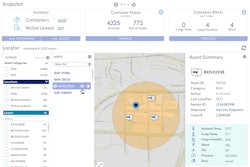
The goal for each session is to refine the specific industry needs resulting in a defined scope and activity level for each topic below.We hope you can join the meetings and actively participate in the discussions. At the end of the discussions the ISTA Pharma Committee will ask industry members to volunteer their time to participate in the specific activities for each topic.
Roundtable discussion topics: