Not so fast, counters Dennis Furniss, Partner and Executive Vice President at
Kaleidoscope Branding. Furniss still sees some fundamental cracks in the armor.
Foremost, he believes, both CPG companies and retailers have overly complicated the national brands vs. store brands issue. National brands on the whole portray themselves as category masters while many retailers continue to focus their own brands primarily as the lower-priced alternative.
Furniss believes both sides should concentrate more on providing both tangible and intangible benefits that are plausible to consumers. "Don't be a brand duplicator or replicator. Is it just on price? Then I'm not buying into value," he says.
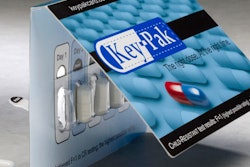
One new branding strategy to watch is for A&P's Live Better brand. The grocer has adopted a positioning of healthful living as a marketing strategy for its pharmacy department. Live Better, a new own brand spanning more than 1,000 SKUs across multiple products, ties directly into this marketing concept.
The Live Better lineup of OTC pharmaceutical and beauty products elevates lifestyle considerations over price, says Todd Maute, Senior Vice President and Partner at
CBX Strategic Branding, which created the design.
Packaging is vital in communicating the lifestyle message. The labels emphasize the Live Better brand rather than identifying the brand as an A&P offering. The Live Better logo forms a banner stretching across a sky-blue background and enhances the brand's efficacy message. The design unifies and identifies the individual products in the Live Better family so consumers can find them easily in categories that are crowded and complex to shop.
Second, retailers need to make smarter category decisions. Furniss still sees a lot of questionable effort being expended in creating low-cost alternatives to national brands that clearly dominate in some categories.
Furniss advises that retailers would do well to instead focus on business models such as Tesco's. The UK-based international grocery giant identified gaping holes in the wine department within its stores, and it has taken category ownership with its own brands through tactics such as forging a partnership with E.&J. Gallo Winery. Consequently, Tesco has earned a reputation in Europe as a destination retailer for wine.
Finally, Furniss mentions the need to create more intelligent shopping experiences. Consumers shop in good/better/best mode, but in Furniss' experience working with both national and store brands, brand managers make mistakes when they don't thoroughly understand what consumers are willing to trade up to within their category.
"And some retailers also are confrontational when we talk to them," Furniss observes. "They are into vertical thinking versus horizontal thinking. They need to think a little more. What are the opportunities to build across a category, rather than merely adding an alternative product?"
So it appears that heading into 2010 and beyond, the stage is set for some healthy competition on a more sophisticated level between retailers' brands and national brands. However, while retailers clearly are leveling the playing field in package design, significant gaps continue to remain between consumers' desire to shop categories horizontally and brand owners' penchant for presenting their product offerings more vertically.
Kaleidoscope BrandingCBX Strategic Branding