At TraceLink’s FutureLink 2018 event held last November, Abbott’s Cyndi Poetker and Biogen’s Bjoern Rosner indicated their companies were looking at ways to gain a return on investment from their global serialization efforts. That approach to actually pursue benefits still seems ahead of the curve compared to so many companies that lament considerable financial loss from the investment—not to mention its role in reducing productivity and output.
Healthcare Packaging’s exclusive Serialization Playbook survey looked at the role of serialization on packaging lines from multiple points of view. The Playbook was downloaded more than 4,700 times between 2012 and 2018. Each person answered a series of questions that revealed details such as where they worked, their role in serialization, as well as key metrics such as how many lines they were responsible for serializing and where those lines were located.
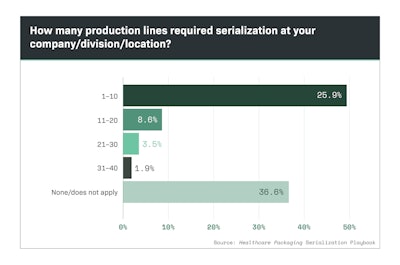
Nearly half of survey respondents reported their companies serialized between one and 10 production lines, as shown in Chart 5.1. Chart 5.2 shows that 53% of respondents had to serialize production lines at one to five physical plant locations.
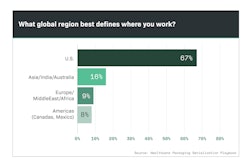
An especially interesting finding came from the question, “What percentage of your lines requiring serialization were in the U.S.? Although one-quarter of respondents said that number was 71% to 100%, nearly half said none of their lines demanding serialization were in the U.S.
Dirk Rodgers, Founder of RxTrace.com, responded by saying, “The fact that nearly half of Playbook readers indicated that none of the packaging lines being retrofitted with serialization capabilities were in the U.S. is likely a reflection of how much of our drug supply is being manufactured and packaged offshore. But these results may also be influenced by the need for serialization for other markets outside of the U.S.”