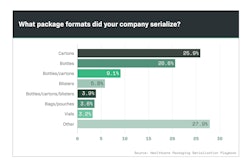
No one really knows how artificial intelligence will affect culture and society, but there’s no shortage of theories. From increased productivity to doomsday scenarios, every outcome has been explored in sci-fi movies. A recent Stat News article said the FDA wants to take some guesswork out of the equation by creating greater oversight over the quickly evolving segment of AI products in medicine that constantly change based on exposure to new patients and data.
Scott Gottlieb, the current (but not for long) commissioner of the FDA, released a white paper that outlined the agency’s approach. The main thesis of the paper is to establish when AI-driven medical products will need FDA review before they’re commercialized. A review may look at the underlying performance of a product’s algorithms, a manufacturer’s plan for modifications, and the manufacturer’s ability to manage the risks that come with them.
“Artificial intelligence has helped transform industries like finance and manufacturing, and I’m confident that these technologies will have a profound and positive impact on health care,” Gottlieb wrote. “I can envision a world where, one day, artificial intelligence can help detect and treat challenging health problems, for example by recognizing the signs of disease well in advance of what we can do today.”