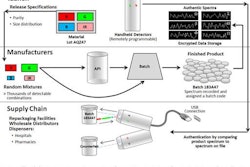
The FDA will require all new drug and biologic product submissions to be filed electronically within the next two years, reported the Regulatory Affairs Professionals Society.
In 2003, the FDA adopted the International Conference on Harmonisation's electronic common technical document, better known as eCTD. It has been accepting electronic applications since the, but now, it will be the only way to do it.
By doing this, it makes it easier for a company to submit an application to multiple regulation agencies, because the eCTD is used across the world, according to the report.