Packaging World/Healthcare Packaging legal columnist Eric F. Greenberg reports, “The U.S. Food & Drug Administration, which has rejuvenated its enforcement program in a variety of ways, has issued 22 Warning Letters to operators of Web sites the agency claims are selling drugs that are unapproved or misbranded to people in the U.S.”
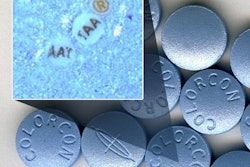
As part of its effort, the FDA purchased and analyzed several products represented online as Tamiflu (oseltamivir). An FDA press release noted: “One of the orders, which arrived in an unmarked envelope with a postmark from India, consisted of unlabeled white tablets taped between two pieces of paper. When analyzed by the FDA, the tablets were found to contain talc and acetaminophen, but none of the active ingredient oseltamivir.”
On a related matter, in-Pharma Technogist.com reported “a five-day international anti-counterfeiting operation involving regulators, police and customs officials from 24 countries has resulted in the seizure of 167,000 counterfeit pills.”
As part of its effort, the FDA purchased and analyzed several products represented online as Tamiflu (oseltamivir). An FDA press release noted: “One of the orders, which arrived in an unmarked envelope with a postmark from India, consisted of unlabeled white tablets taped between two pieces of paper. When analyzed by the FDA, the tablets were found to contain talc and acetaminophen, but none of the active ingredient oseltamivir.”
On a related matter, in-Pharma Technogist.com reported “a five-day international anti-counterfeiting operation involving regulators, police and customs officials from 24 countries has resulted in the seizure of 167,000 counterfeit pills.”