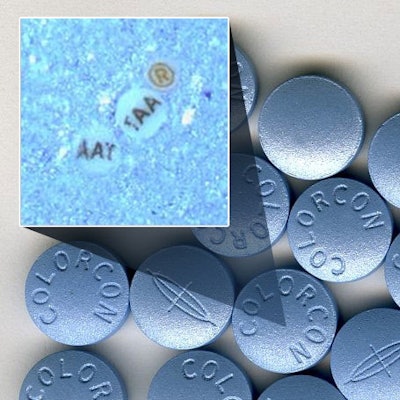
The alliance provides Colorcon exclusive rights to market ARmark's micro-tags for tablet film coatings and to market this technology to pharmaceutical manufacturers globally.
By leveraging the tablet coating expertise of Colorcon, a manufacturer and developer of specialized film coatings, and ARmark's customized micro-tag authentication technology, the two companies collaborated to develop ®mark® On-Dose ID. The micro-tags are integrated into existing tablet film coating processes without additional manufacturing steps or capital investments. By incorporating the micro-tags directly into a tablet's film coating, drug manufacturers achieve reliable placement on each tablet.
The customized micro-tags can hold significant amounts of brand-owner specific, encrypted information such as lot and batch ID numbers, logos and other text, patterns, shapes and symbols, in a particle the size of 75-110 microns, a space smaller than the diameter of a human hair. Said to be nearly impossible to replicate or reverse engineer, the markers are compatible with other covert or overt identification technologies and can also be incorporated as part of labels, bottles, paper and blister packaging.
“The availability of this authentication technology is significant to drug manufacturers because adding ®mark® micro-tags to an existing immediate release film coated tablet can now be considered to be a SUPAC Level 1 annual reportable change,” states Jeff Robertson, director and general manager at ARmark. “That means drug manufacturers can quickly begin using the On-Dose ID technology to protect their product without prior approval from the FDA.”
®mark® covert marker technology has been available for more than two years in other markets, but the recent draft guidance issued by the U.S. Food and Drug Administration (FDA) regarding the use of physical chemical identifiers (PCIDs) defines how the commercial utilization of micro-tag technology can be used for on-dose authentication in the pharmaceutical industry. In accordance to the FDA's guidance document, ARmark manufactures the micro-tags from approved excipient materials generally regarded as safe (GRAS) or from the FDA's Inactive Ingredient Guide (IIG), under cGMP conditions.
According to Dr. Kamlesh Oza, Colorcon's general manager of film coatings, "Colorcon's studies have shown there is no impact on dissolution or stability when the ®mark® On-Dose ID micro-tags are incorporated into an existing film-coated dosage form. This approach is in compliance to the FDA's guidance, enabling pharmaceutical companies to provide patients with safe security augmentation. As companies adopt this anti-counterfeiting technology to secure their brands, our studies will help them note the addition of micro-tags in their annual reports as required by the FDA."
The ®mark® On-Dose ID micro-tags are invisible to the naked eye once they are incorporated into a tablet film coating, but are identified with ®vision® optical viewing systems. These portable tools authenticate products by magnifying the micro-tags at any stage following the tablet coating process. The ®vision® system enables accurate, in-field detection within a matter of seconds without destroying the SODF sample.
By leveraging the tablet coating expertise of Colorcon, a manufacturer and developer of specialized film coatings, and ARmark's customized micro-tag authentication technology, the two companies collaborated to develop ®mark® On-Dose ID. The micro-tags are integrated into existing tablet film coating processes without additional manufacturing steps or capital investments. By incorporating the micro-tags directly into a tablet's film coating, drug manufacturers achieve reliable placement on each tablet.
The customized micro-tags can hold significant amounts of brand-owner specific, encrypted information such as lot and batch ID numbers, logos and other text, patterns, shapes and symbols, in a particle the size of 75-110 microns, a space smaller than the diameter of a human hair. Said to be nearly impossible to replicate or reverse engineer, the markers are compatible with other covert or overt identification technologies and can also be incorporated as part of labels, bottles, paper and blister packaging.
“The availability of this authentication technology is significant to drug manufacturers because adding ®mark® micro-tags to an existing immediate release film coated tablet can now be considered to be a SUPAC Level 1 annual reportable change,” states Jeff Robertson, director and general manager at ARmark. “That means drug manufacturers can quickly begin using the On-Dose ID technology to protect their product without prior approval from the FDA.”
®mark® covert marker technology has been available for more than two years in other markets, but the recent draft guidance issued by the U.S. Food and Drug Administration (FDA) regarding the use of physical chemical identifiers (PCIDs) defines how the commercial utilization of micro-tag technology can be used for on-dose authentication in the pharmaceutical industry. In accordance to the FDA's guidance document, ARmark manufactures the micro-tags from approved excipient materials generally regarded as safe (GRAS) or from the FDA's Inactive Ingredient Guide (IIG), under cGMP conditions.
According to Dr. Kamlesh Oza, Colorcon's general manager of film coatings, "Colorcon's studies have shown there is no impact on dissolution or stability when the ®mark® On-Dose ID micro-tags are incorporated into an existing film-coated dosage form. This approach is in compliance to the FDA's guidance, enabling pharmaceutical companies to provide patients with safe security augmentation. As companies adopt this anti-counterfeiting technology to secure their brands, our studies will help them note the addition of micro-tags in their annual reports as required by the FDA."
The ®mark® On-Dose ID micro-tags are invisible to the naked eye once they are incorporated into a tablet film coating, but are identified with ®vision® optical viewing systems. These portable tools authenticate products by magnifying the micro-tags at any stage following the tablet coating process. The ®vision® system enables accurate, in-field detection within a matter of seconds without destroying the SODF sample.
Companies in this press-release






















