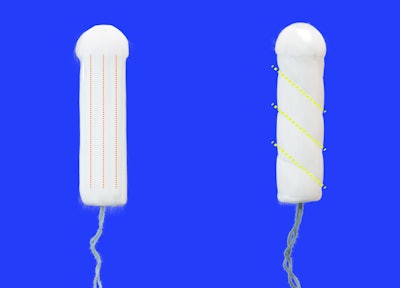
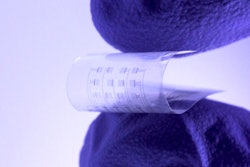
According to a recent Wall Street Journal article, Sequel, a menstrual health startup based in San Francisco, has achieved a significant milestone by obtaining FDA clearance for its novel tampon design. Unlike conventional tampons with linear grooves, Sequel's features spiraled grooves engineered to optimize liquid absorption. While the FDA clearance paves the way for Sequel to introduce its innovative product to the market, the real challenge lies ahead in effectively marketing and competing against established giants in the menstrual care industry, such as Procter & Gamble, Kimberly-Clark, and Edgewell.
Breaking into the menstrual care market has proven to be a formidable task for startups, since the category is marred by habit and preference. Sequel plans to distinguish itself through an unconventional strategy. Rather than relying on traditional advertising, the startup intends to initiate a sampling campaign in select venues associated with wellness and fitness in major cities like New York, San Francisco, and Austin. This approach is aimed at creating a direct and personal experience with potential users, showcasing its tampon's unique benefits and positioning itself as a brand that offers an elevated product experience. This innovative marketing strategy aligns with Sequel's focus on disrupting the status quo and establishing a niche for itself in the highly competitive menstrual care industry.