NEJM
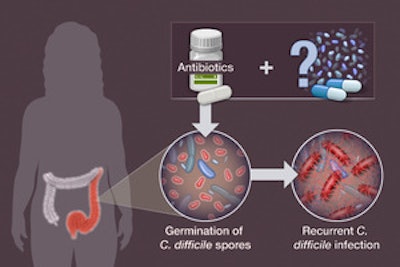
C. diff is a bacterial infection that can cause severe diarrhea and inflammation of the colon, and it often recurs even after treatment with antibiotics. According to a recent LIFE-SCIENCE article, the FDA has approved the first-ever pill made from human feces for the treatment of recurrent Clostridioides difficile (C. diff) infection. The pill, called "SER-109," contains spores from multiple strains of bacteria found in healthy feces that can help restore the gut microbiome and fight off C. diff infections.