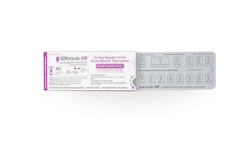
In response to the growing need for light, oxygen, and moisture barrier packaging, Winpak, a global packaging manufacturer, is introducing WinForm 25-45-60 Cold Form laminate for sensitive, hygroscopic pharmaceutical and biopharmaceutical products.
Three-ply, cold-formable WinForm contains a 45-micron foil sandwiched between a 60-micron PVC film and a proprietary 25-micron film that allows the laminate to stretch in three directions for deeper draw cavities without delamination, cracks, and other stresses. While providing deeper draw cavities, WinForm 25-45-60 still optimizes machine speeds due to the added strength and flexibility of the blister laminate.
Winpak also offers WINSAFESM Technical Services, which assists customers in ensuring proper tooling design and forming of WinForm 25-45-60 with no impact on barrier properties. Winpak will be exhibiting WinForm 25-45-60 at Interphex 2015, April 21- 23 in New York City, Booth #1212.