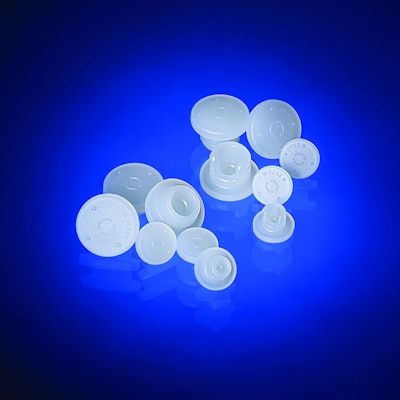
West Pharmaceutical Services, Inc., a global developer and manufacturer of components and systems for injectable drug delivery, and partner Daikyo Seiko, Ltd., Tokyo, Japan, have announced the commercial launch of the Daikyo D4 elastomer formulation.
To meet the ever-increasing quality demands for sensitive drug products, Daikyo Seiko developed the all-new elastomeric formulation. Daikyo’s D4 13mm and 20mm serum and lyophilization stopper configurations will be available as 100% vision-inspected, Ready-to-Sterilize (RSV) quality from Daikyo Seiko, as well as in sterile Ready-to-Use (Westar® RU) quality, as part of the West Ready Pack® system.
The light and translucent color of Daikyo D4 creates an aesthetic appearance. Daikyo D4 allows enhanced visual inspection performance for particulate burden, and can help reduce final drug reject rates due to particulate. Striving for the highest in drug compatibility, chemical purity and increased risk mitigation, all Daikyo D4 stopper configurations are laminated with Flurotec® barrier film.
Daikyo D4 requires shorter drying times for lyophilization applications, and can help to shorten component preparation times. Daikyo D4 is free of sulfur and zinc, which are commonly used curatives in elastomeric formulations that may cause sensitivity in biologics.
The new Daikyo D4 stopper configurations are compliant with Japanese, U. S., and European compendia requirements in all offered configurations.
Daikyo D4 is for:
• Aqueous drug products that might be sensitive to extractables and leachables
• Lyophilized products that could benefit from low-moisture formulations that lead to shorter drying times
• Drug products in need of an aesthetically clean packaging solution
• Drug products intended for global markets
Steam-sterilized stoppers manufactured from the Daikyo D4 formulation are available as part of the West Ready Pack® component system, which offers a combination of ready-to-use vials, stoppers and seals with proven container closure integrity, as well as vial adapters.