Mavenclad / Image: Social MedWork
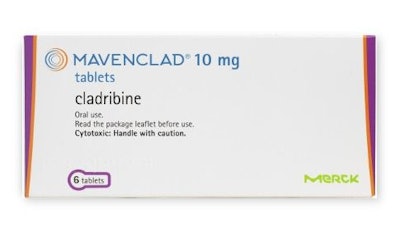
A recent FDA News Release article noted the FDA’s approval of cladribine tablets that treat relapsing forms of multiple sclerosis. The drug, which goes by the brand name Mavenclad, was approved for EMD Serono, the biopharmaceutical business of Germany-based Merck KgaA.
In a clinical trial conducted with over 1,300 patients, Mavenclad significantly decreased the number of relapses and reduced the progression of disability compared to a placebo.