“Keeping the U.S. Prescription Drug Supply Chain Among the Safest in the World,” was the headline of a July 20 blog by Ilisa Bernstein, Pharm.D., J.D., and Deputy Director of the Office of Compliance in FDA’s Center for Drug Evaluation and Research.
A story on the agency’s website explains that a global approach was taken to create a “Supply Chain Security Toolkit for Medical Products” that can serve as a resource for “industry stakeholders and regulators worldwide to adopt best practices, for training purposes, and to strengthen laws and regulations to protect consumers from unsafe and substandard drug products was the subject.”
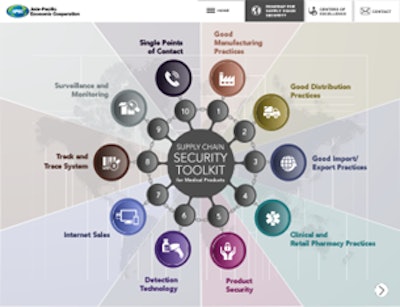
FDA also referred to the tookit as “a roadmap to promote global medical product quality and supply chain security.” The tookit provides training materials in the following 10 categories:
• Good Manufacturing Practices
• Good Distribution Practices
• Good import/export practices
• Clinical/retail pharmacy practices
• Product security
• Detection technology
• Internet sales
• Track-and-trace systems
• Surveillance and monitoring
• Single points of contact
In her blog, Bernstein noted, “Substandard and falsified drugs are global problems that need global solutions and global collaboration. We cannot solve these challenges alone and we at FDA are continually looking for ways to collaborate and learn from our regulatory counterparts aroundthe world. … Together with our global partners, we will continue to combat supply chain problems as they arise and increase confidence in the legitimacy of the life-saving prescription drugs that patients rely on.”