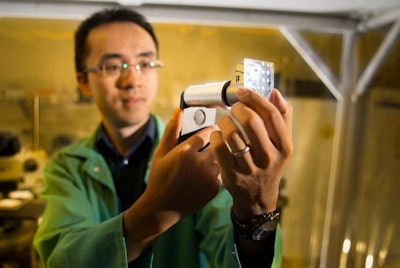
Whether they have no active ingredient, the wrong ingredient, or just the wrong dosage, imposter drugs take nearly a million lives a year. Chemist Jun Wang may have found a solution to the global issue of drug fraud. A July 29th Smithsonian article discusses Wang’s idea to create QR codes tiny enough to imbed in drugs or print on capsules, yet still readable by cell phones.
The tiny codes are created via photolithography, and can be read with the aid of a $10 cell phone microscope. “The material itself is very, very inexpensive, and the procedure for making QR bar codes is very standard in the industry, so I don’t think the price would be high,” says Wang.
Wang and his team hope to develop a specific app to read the codes, and team up with a pharmaceutical company to bring the concept to market in the next few years. Due to the edible nature of the codes, the application spans far beyond pharmaceutical use and can be applied to expensive and commonly counterfeit foods, such as Kobe beef and Parmesan cheese.