A July 14 U.S. Consumer Product Safety Commission report advised the following: “Consumers should stop using this product unless otherwise instructed. It is illegal to resell or attempt to resell a recalled consumer product.”
Panadol Advance 100-count caplets pose a hazard, said CPSC, as “the packaging is not child-resistant as required by the Poison Prevention Packaging Act. These products contain acetaminophen, which is required by the Poison Prevention Packaging Act to be sealed with child-resistant packaging.
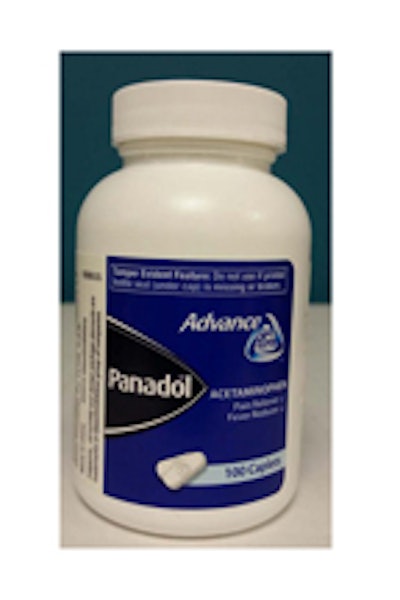
CPSC said the recall involves about 10,600 units and “was sold in white containers with a blue label, inside a blue box. ‘Panadol’ and ‘Advance’ are printed on the label. Lot numbers and date codes are located on the left-side panel of the box and on the left side of the label on the bottle, near the barcode.” CPSC said lot numbers and date codes included in the recall are:
• Lot number: 14241, expiration date: 02/2015
• Lot number: 14002, expiration date: 10/2014
• Lot number: 13881, expiration date: 09/2014
• Lot number: 13801, expiration date: 09/2014
No incidents or injuries were reported, but CPSC said, “Consumers should immediately place the product out of a child's sight and reach, and contact GSK for a refund.”
The affected product was sold exclusively at drug, grocery, and mass merchandise stores in Puerto Rico from Nov. 2012 through Feb. 2014 for about $10.
GSK announcement
GSK announced on July 9 that it was voluntarily recalling all “all Panadol® Advance 100-ct pain relief products from Puerto Rico consumers.” A press release on its website said the following:
“Panadol® Advance 100-ct contains the ingredient acetaminophen (paracetamol), and thus the bottle requires sealing with a child-resistant feature or to be labeled as a product ‘for households without young children.’ Because the product does not have a child-resistant cap, there is a risk of unintentional ingestion and overdose.
“The health and safety of our consumers is our first priority, and while the product is safe to use as directed, we ask consumers to please keep the product away from children and contact us to return any bottles of Panadol® Advance 100-ct they may have,” said Juan Arias Camisón, General Manager for GSK Consumer Healthcare in Puerto Rico.
“We urge consumers to contact the dedicated line for the Panadol® Advance 100 ct recall at 1-888-912-8455, from 8:00 AM to 5:00 PM, for instructions on how to return this product for a refund.”





















