Counterfeit drugs have been a major hurdle for pharmaceutical companies worldwide—approximately 73 billion euros of counterfeit medicine are distributed annually, according to the World Health Organization.
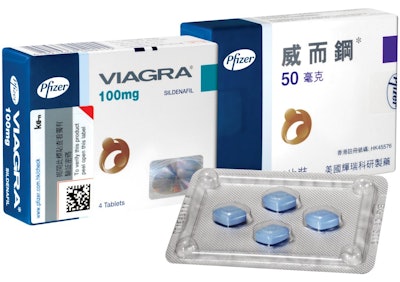
Pfizer, a corporation that develops and produces medicine and vaccines for numerous medical disciplines, found that sales of their blockbuster drug Viagra were being undermined in Asian markets by major counterfeiting operations. In Hong Kong alone, approximately 40% of Viagra sales were found to be counterfeit.
To expose the fakes and increase the sales of the authentic drug, Pfizer turned to Kezzler’s serialization technology, which is designed to protect brands, track distribution, and strengthen consumer loyalty in the pharmaceutical industry using Kezzlercodes. Pfizer currently uses Norway-based Kezzler’s serialization technology to protect eight of its biggest brands.
“Aggregation for track and trace is the next level, but in this case a quick and fast solution to tackle the problem of counterfeit Viagra led to the prioritization of the serialization solution,” says Christine C. Akselsen, CEO of Kezzler.
Kezzler applied both visible and non-visible codes, which were unique, secure, and traceable, to each pack of Viagra at the distribution centers, avoiding packaging lines completely to speed the serialization process. Full implementation took place within a few weeks of the printing and approval of the first batch of labels.
The codes enabled consumers, Pfizer, and other supply chain stakeholders to verify every pack’s authenticity with a scanner, but a major challenge was educating consumers on how to use the new security feature. However, Kezzler introduced QR and Datamatrix codes to the package design, allowing consumers to use a smartphone to verify authenticity in a matter of seconds.
“A well-executed serialization program provides effective protection, especially when it arms the patient with a very powerful tool that enables them to authenticate their product directly and in real-time,” says Akselsen. “Following rollout, customers can place their trust in three parties: Pfizer, the Kezzler system, and themselves by inspecting the product directly.”
Kezzler’s rapid response led to a substantial increase in sales of the authentic Viagra, according to Kezzler.
This project launched in 2012 and has since expanded to several more products and countries in Asia, for which the Pfizer team also won a Commercial Innovation Award. Currently, Kezzler has integrated their online system with point of sales systems at pharmacies in Hong Kong so that every sale is now reported and documented for Pfizer.