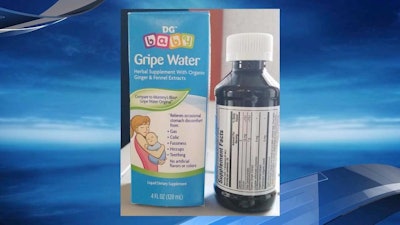
Gripe Water / Image: KATU
A February 19th Today article noted the recall of DG Baby Grip Water, an herbal water supplement for babies. According to the FDA recall notice, the product was pulled from Dollar General stores “due to the presence of an undissolved ingredient, citrus flavonoid.” The product contains organic ginger and fennel extracts and is intended to relieve stomach discomfort from gas, colic, hiccups and teething.
Kingston Pharma, the manufacturer of the product, received a report that a week-old infant had difficulty swallowing the product, and three more complaints about the undissolved ingredient.
Companies in this article