This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
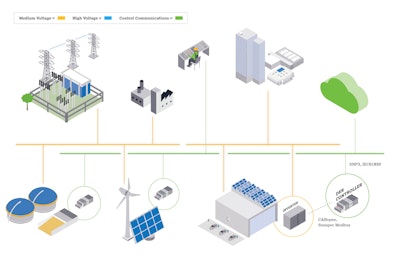
The new Distributed Energy Resources (DER) Controller from WAGO is a library function block with a control algorithm for voltage, frequency, and reactive power control. To ensure a reliable power system, the DER connection to the grid requires communication between the energy source and the operator. Standards like IEEE 1547-2018 have been implemented to provide a trouble-free connection between systems.
Of the 11 clauses related to IEEE 1547-2018, WAGO’s DER supports clauses 5 and 10. Clause 5 deals with reactive power and voltage/power control. This reinforces Clause 10, which handles the subject of interoperability for information exchange. The library function block in these controllers is programmed with CODESYS 3.5 software and has been validated and verified by a Nationally Recognized Testing Laboratory.





















